Research
Neuropeptide and peptide hormone biosynthesis; secretory vesicle biogenesis and trafficking.
Research in this laboratory focuses on the regulation of biosynthesis, processing, intracellular trafficking, and secretion of neuropeptides and peptide hormones in neuronal and endocrine cells with an emphasis on the cellular organization of these biological processes. Neuropeptides and peptide hormones are synthesized from larger multivalent precursors, and the biosynthesis may be regulated at the transcriptional, post-transcriptional (RNA splicing), translational, or post-translational levels. Through these regulatory mechanisms, expression of the same gene coding for a particular precursor can result in a different set of related processed peptides in different cells. The peptides secreted ultimately dictate the identity and function of that peptidergic cell. Such regulatory mechanisms and the cellular components that make up the biosynthetic and regulated secretory pathway are studied. A recent major focus of the laboratory has been on the mechanisms by which neuropeptides, peptide hormones, and their processing enzymes are sorted at the trans-Golgi network and targeted to the regulated secretory pathway and post-Golgi transport of hormone containing vesicles to the release site. Mechanisms involved and under study include novel conformation dependant sorting signals, sorting receptors and cholesterol-rich lipid microdomains (rafts). The aim of this research is to understand molecular mechanisms of biosynthesis and sorting of proteins to the regulated secretory pathway in peptidergic neurons and endocrine cells in normal and disease states. Such studies have provided an insight into the molecular basis of diseases such as familial hyperproinsulemia and obesity which result from intracellular missorting of proinsulin and cocaine amphetamine regulated transcript (CART) respectively to the constitutive pathway.
These studies are conducted using a multidisciplinary approach. Whole animals, "knock-out " mice, fresh tissues and organs, and dissociated cell cultures are analyzed using various biochemical, pharmacological, immunological, and recombinant-DNA techniques. These include protein and peptide separation, enzymology, radioimmunoassay, immunocytochemistry, confocal microscopy, recombinant-DNA methodology, and in situ hybridization.
- Carboxypeptidase E is a Sorting Receptor for the Regulated Secretory Pathway
- Diseases Related to the Sorting of Prohormones
- Sorting and Processing of Prohormones in the Regulated Secretory Pathway
- Sorting of Processing Enzymes to the Regulated Secretory Pathway: œ-Helices and Lipid Rafts
- Secretory Granule Biogenesis
- Secretory Granule Trafficking
Trophic Roles of CPE /Neurotrophic factor-α1
We have discovered non-enzymatic, trophic roles of CPE/NF-α1 in neuroprotection during stress, embryonic neurostem cell differentiation and regulation of bone mass and bioenergetics of skeletal stem cells.
CPE /NF-α1 in neuroprotection and anti-depression
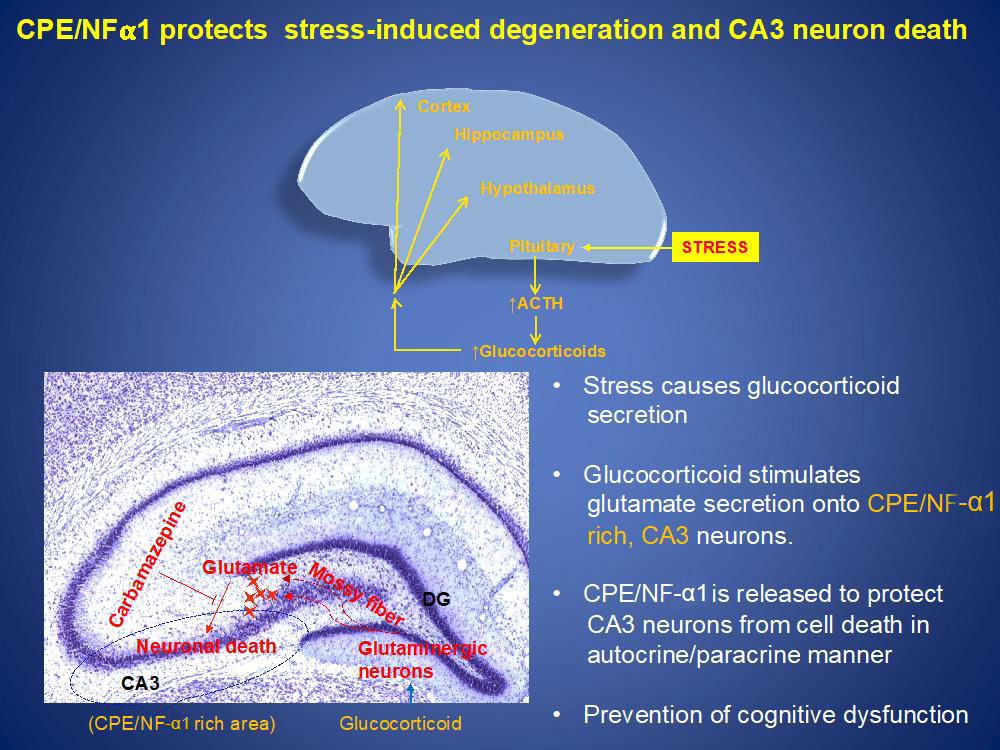
During stress, glucocorticoids are secreted from the adrenals into the circulation. Glucocorticoids then goes to various areas of the brain including the hippocampus where it stimulates glutaminergic neurons to secrete glutamate onto CPE/NF-α1 rich neurons in the CA3 region. These neurons then secrete CPE/NF-α1 to protect them from glutamate- induced cell death in an autocrine/paracrine manner. Since CA3 neurons are very important in cognitive function, absence of CPE/NF-α1in CA3 neurons resulted in their degeneration and cognitive dysfunction in CPE-KO mice after stress. Indeed, a human with a CPE-null mutation showed cognitive dysfunction. Furthermore, we showed that the neuroprotective effect is independent of CPE-enzymatic activity as evidenced by the observation that a CPE-E342Q knock-in mutant mouse that lacks CPE enzymatic activity showed neuroprotection of CA3 neurons against severe stress.
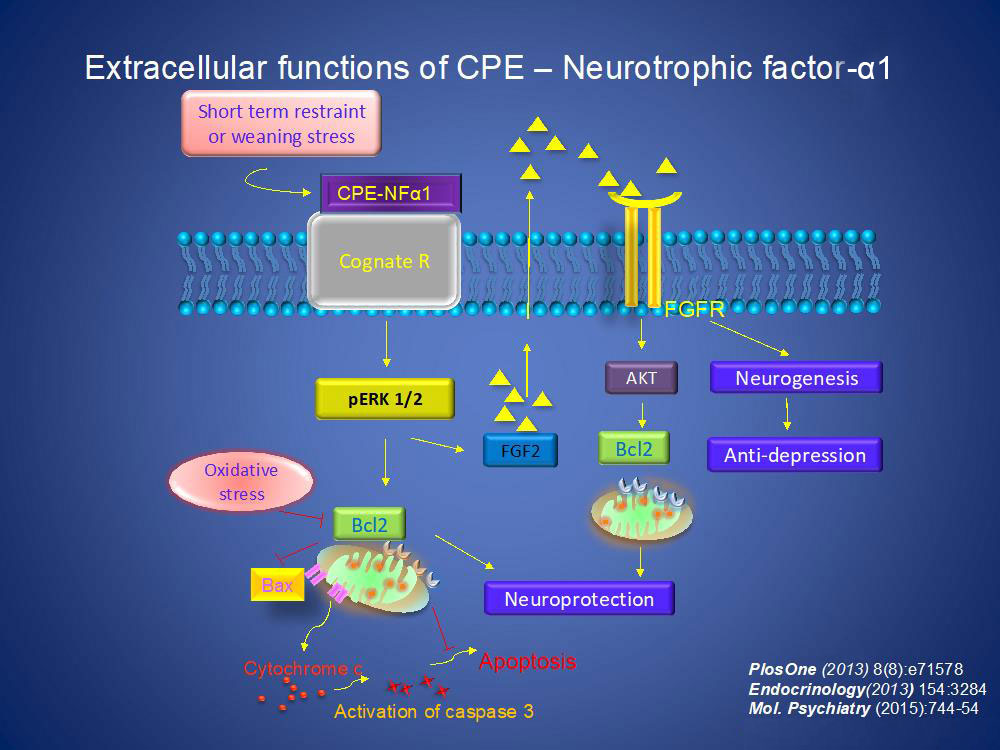
At the cellular level, CPE/NF-α1 binds to a cognate receptor and activates ERK pathway to increase expression of the pro-survival protein BCL2 to mediate neuroprotection. CPE-NF-α1 also stimulates FGF2 expression which can in turn stimulate neurogenesis and anti-depression. Recently, we have identified a human GPCR serotonin receptor, HTR1E, that interacts with CPE/NF-α1 resulting in ERK-signaling and protection against oxidative-stress induced cell death via BLC2 activity.
CPE /NF-α1 and CPE-ΔN in embryonic neurodevelopment
CPE-KO mice exhibit developmental deficits such as abnormal dendritic arborization and spine morphology, and neonates were significantly impaired in almost every test of strength and co-ordination and motor performance suggesting that CPE/NFα1 plays a role in neurodevelopment. We have cloned three forms of CPE-NFα1 in embryonic mouse brain, with sizes 2.3, 1.9 and 1.73 kb. Among the three transcripts, the major one was ~2.3kb in size, which correlated with WT-CPE (53kD protein); the other two smaller transcripts, present in lower amounts were identified as novel N-terminal truncated CPE transcript variants. The 1.9 kb and 1.73 kb mRNAs encode a 47kD and a 40kD CPE/NFα1-ΔN protein, respectively, with the latter being more abundant. WT-CPE is expressed abundantly in embryos and adult brain, but undetectable in other organs. CPE-NFα1-ΔN was present in embryonic but not adult brain. Expression of the 3 mRNAs peaked at embryonic day E10.5 correlating with initiation of neurogenesis in developing brain. A second surge occurred at post-natal day 1 (P1), a time of massive astrocytogenesis and synaptogenesis.
We studied the effect of CPE-NFα1 on stem cell proliferation and differentiation using the neurosphere/neural stem cell model. Recombinant CPE-NFα1 added to E13.5 neocortex-derived neurospheres, reduced proliferation by 41%. Since CPE-NFα1 interacts with Wnt-3a ligand binding receptor (Frizzled) and negatively regulates β-Catenin expression, we investigated whether β-Catenin and its downstream molecules known to regulate cell proliferation are involved. NF-α1 treated neurospheres, showed a decrease in β-Catenin and Hes1, with no change in Cyclin D1 protein level, indicating that NF-α1 negatively regulates cell proliferation in neurospheres via Wnt signaling. To assess the role of NF-α1 in neural stem cell (NSC) differentiation, neurospheres from 7d cultures were dissociated and grown for 5d in the presence of NF-α1. Analysis of differentiated cells labeled with specific antibodies against neurons (Tuj-1), astrocytes (anti-GFAP) and oligodendrocytes (anti-CNPase) showed ~1.4-fold increase in GFAP+ cells in the presence of NF-α1, with a trend towards a decrease of Tuj-1+ and no change in the CNPase+ populations. Double immunostaining against nestin (anti-nestin) and astrocytes revealed the increase of the GFAP+ cell population upon NF-α1 treatment comes from the intermediate glia population (GFAP+ nestin+ cells). This data indicated that CPE/NF-α1 induced differentiation of embryonic NSC/progenitors to astrocytes. Furthermore we demonstrated that CPE-NFα1 activated ERK-signaling in NSCs which then increased the level of GFAP via the transcription factor Sox9, a mechanism known to be involved in astrocyte differentiation. NSC/progenitors from CPE-KO mouse embryos showed ~1.4-fold fewer GFAP+ cells and ~1.5-fold more Tuj-1+ cells compared to CPE-NFα1-WT controls. This difference was rescued upon treatment of NCS from these mice with recombinant NF-α1. In vivo, immunocytochemistry of brain sections and western blot analysis of neocortex of mice showed a gradual increase of CPE-NFα1 expression from E14.5 to P1 and a surge of GFAP expression at P1, the time of increase in astrogenesis. Importantly, NFα1-KO mice showed ~49% fewer GFAP positive astrocytes in the neocortex compared to WT mice at P1. Thus, CPE-NFα1 is critical for regulating anti-proliferation and cell fate determination, through differentiating embryonic stem cells to GFAP-positive astrocytes for normal neurodevelopment.
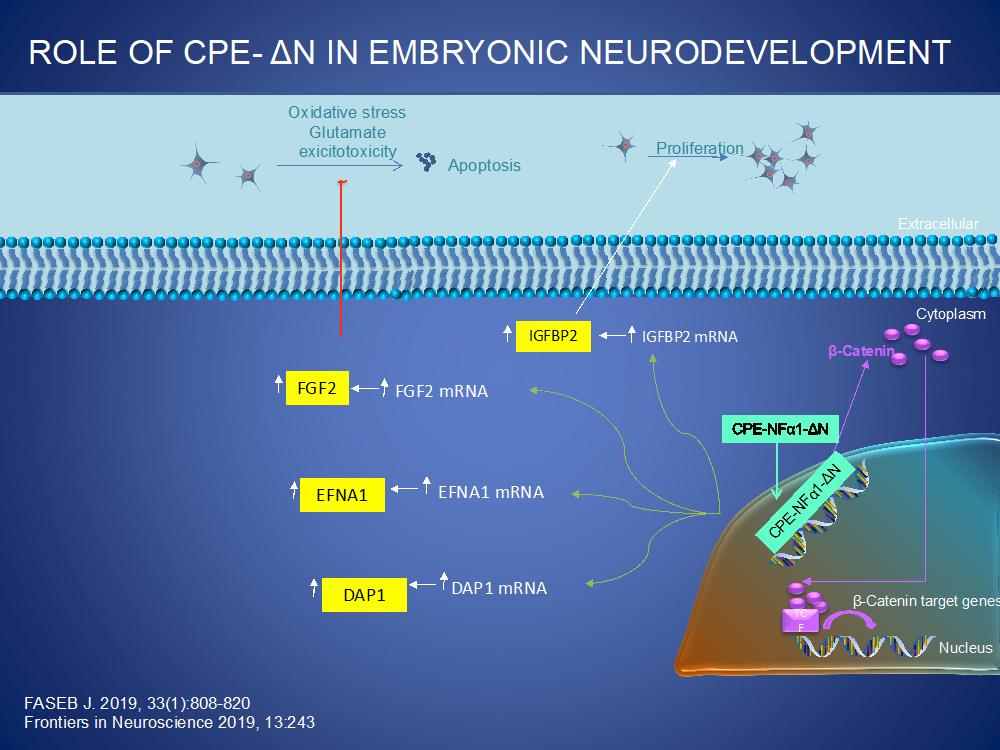
CPE/NFα1-ΔN also plays a significant role in neurodevelopment. As illustrated below, CPE/NFα1-ΔN is transported from the cytoplasm into the nucleus where it up-regulates the transcription of various genes that are involved in proliferation, (IGFBP2, β-catenin), neuronal migration, (EFNA1) and programmed cell death, (DAP1). CPE/NFα1 also upregulates the expression of FGF2 in embryonic neurons to mediate proliferation and neuroprotection against oxidative stress and glutamate excitotoxicity.
CPE /NF-α1 in regulation of bone mass and energy metabolism
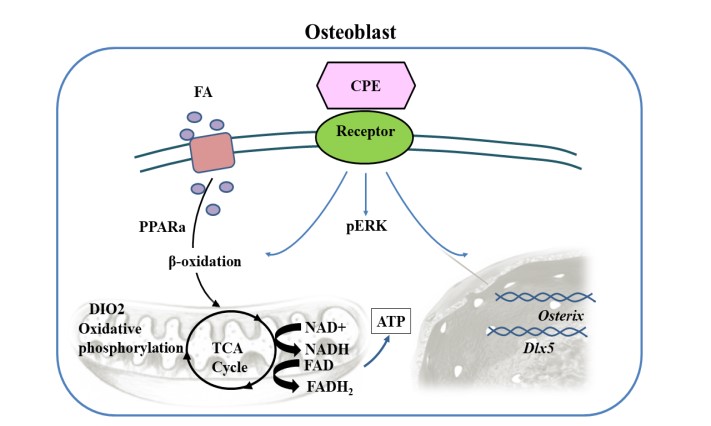
Collaborating with Dr. Beata Lecka-Czernik (U. Toledo), we observed that CPE-NFα1 expression in primary human mesenchymal cells cultivated from iliac crest marrow aspirates increased up to 30-fold during osteogenesis, suggesting a possible role of CPE-NFα1 in bone formation. Compared to WT animals, CPE-KO mice have low trabecular and cortical bone mass in both appendicular and axial skeleton, and increased volume of marrow fat. While metabolic deficit in CPE-KO mice developed early in life, bone deficit developed in older age. Eight-week old CPE-KO males are obese and have high bone mass, whereas 40-wk old males are still obese but have very low bone mass. Interestingly, CPE bone-specific activity differs from its enzymatic activity. Mice carrying knock-in mutant CPE-E342Q lacks enzymatic activity and develop the same obese phenotype as CPE-KO mice. However, their bone mass was not different from WT animals. An analysis of CPE-KO mouse marrow mesenchymal stem cells (MSCs) showed increased potential to adipocyte differentiation, which is not affected in MSCs carrying E342Q mutation, and there was no defect in osteoblast differentiation measured at the levels of gene expression and in ex vivo mineralization assay. Murine marrow MSCs treated with exogenous recombinant CPE or CPE-E342Q protein enhanced Erk phosphorylation and up-regulated expression of two markers of the wnt pathway Cxn43 and Axin2, and a tendency to increase Osterix, a gene associated with osteoblastic differentiation; as well as genes associated with fatty acid metabolism and energy dissipation such as Dio2, exhibiting accumulation of small quantities of lipid droplets in these cells. These findings indicate that CPE-NFα1 besides acting extracellularly as a novel trophic factor in the nervous system, also acts as a tropin to regulate both bone mass and energy metabolism, independent of its enzymatic activity. https://doi.org/10.1002/jbm4.10392
Role of CPE in Cancer
Expression of CPE and CPE-α1 promote tumorigenesis
We have cloned and characterized 2 forms of CPE mRNA from human cancer cells: human wild-type (WT, ~2.4kb) and a 40 kD N-terminal truncated splice variant, CPE-ΔN (~1.7kb), which has 198 nucleotides removed within the first exon and 589 nucleotides from the 3’-UTR, compared to WT-CPE mRNA, in human hepatocellular carcinoma cells (HCC). Both these transcripts were found in other human cancer cell lines such as CAOV3 (ovarian), LN-18, U118 (glioblastoma) and A549 (lung) and in HCC cancer patients. While the WT-CPE is secreted, 40kD CPE-ΔN was found in cytoplasm and nucleus in HCCH and CAOV3 cells. Overexpression of this 40kD CPE-ΔN variant up-regulated expression of multiple metastatic genes encompassing different signaling pathways, suggesting potentially an important role of CPE-ΔN in tumor metastasis. Additionally, suppression of CPE and 40kD CPE-ΔN expression using CPE siRNA that inhibits both indiscriminately, resulted in inhibition of proliferation and invasion of Panc-1 cells, a pancreatic cancer cell line. Expression of CPE-WT or 40kD CPE-ΔN in approximately equal protein amounts in Panc-1 cells led to significant enhancement of proliferation, but only cells transfected with 40kD CPE-ΔN Panc-1 cells promoted invasion. Panc-1cells overexpressing 40kD CPE-ΔN showed an increase in CXCR2 expression which when suppressed by si-RNA significantly decreased proliferation and invasion. Our findings suggest that CPE-ΔN may play an important role in promoting pancreatic cancer growth and malignancy through up-regulating the expression of the metastasis-related gene, CXCR2. The mechanism of action of CPE in proliferation involves regulation of cell cycle via cyclin D1. Our studies and those of others have provided a body of data from cell, animal and clinical patient studies indicating that CPE and 40kD CPE-ΔN promote tumorigenesis in different cancer types and is a potential therapeutic target for treating cancer.
Exosome-based CPE confers and CPE-shRNA loaded exosomes inhibit tumorigenesis.
Exosomes represent a class of membrane bound nano-sized extracellular vesicles (30-140nm in diameter) which are secreted by most cells and are associated with various physiological processes. Exosomes carry biomolecules (proteins, DNA, mRNA and miRNA) unique to their cell of origin and deliver them to recipient target cells thereby mediating cell-cell communication. We have explored the ability of exosomes from high metastatic HCCH cells to confer growth and metastatic properties to HCCL (low metastatic) cells. Additionally, we have explored CPE mRNA copy numbers in human sera exosomes as a possible biomarker for cancer. Exosomes were isolated from the supernatant culture media of cancer cells and a correlation was found between elevated CPE mRNA levels in exosomes from high vs low metastatic cell lines across various cancer types. CPE copy numbers were also significantly higher in sera exosomes from cancer patients compared to controls. Content analysis of exosomes derived from highly metastatic HCC97H cells revealed CPE-WT mRNA and protein. We showed that exosomes released from HCC97H cells were able to enhance invasion and proliferation of HCC cells with poor metastatic ability (HCC97L). However, when CPE expression was suppressed in the HCC97H cells before exosome isolation, the exosomes had no effect on proliferation and invasion. These data demonstrate the ability of exosomes to confer metastasis in cancer cells and the role of exosomal CPE in driving the process. We then utilized the inherent property of exosomes to act as efficient delivery tools to carry a therapeutic agent such as shRNA. Previously it was shown that down-regulation of CPE expression by shRNA can reverse tumor growth and metastasis in an HCC mouse model. We loaded CPE-shRNA into exosomes by infecting HEK293 (Human Embryonic Kidney) cells with adenovirus carrying CPE-shRNA-GFP. These modified exosomes were harvested from the cell medium, purified and then used to transfer CPE-shRNA to HCC97H cells. The exosomes taken up by the recipient HCCH cells resulted in a 4.7-fold reduction of CPE mRNA levels along with ~3.3-fold decrease in proliferation and ~5-fold down-regulation of colony forming ability. We also observed a down-regulation of the transcription factor c-Myc mRNA and Cyclin D1 mRNA and protein required for cell cycle G1/S transition, consistent with decrease in proliferation of the treated cells. These studies demonstrate the possibility of using exosomal CPE as a serum biomarker to detect cancer; and the ability of these exosomes to enhance invasion in low metastatic HCC cells, as well as the potential to use shRNA loaded exosomes to target CPE as a therapeutic strategy to treat liver and other cancers.

 BACK TO TOP
BACK TO TOP