Home
Head: Mary Lilly, Ph. D.
The long-term goal of our laboratory is to understand how the cell-cycle events of meiosis are coordinated with the developmental and metabolic events of oogenesis. Chromosome mis-segregation during female meiosis is the leading cause of miscarriages and congenital anomalies in humans. Recent evidence suggests that many meiotic errors occur downstream of anomalies in the signaling pathways that control oocyte growth and differentiation. Thus, an understanding of how meiotic progression and gamete differentiation are coordinated during oogenesis is essential to studies in both reproductive biology and medicine.
In mammals, studies on the early stages of oogenesis face serious technical challenges in that entry into the meiotic cycle, meiotic recombination, and the initiation of the highly conserved prophase I arrest all occur during embryogenesis. By contrast, in Drosophila these critical events of early oogenesis all take place continuously within the adult female. Easy access to the early stages of oogenesis, coupled with available genetic and molecular genetic tools, makes Drosophila an excellent model for studies on meiotic progression and oocyte development.
The SEA/GATOR Complex
Our lab uses Drosophila melanogaster as a model to explore how metabolism influences the process of oogenesis. Target of Rapamycin Complex 1 (TORC1) is a primary regulator of cell growth and metabolism that responds to multiple signals including nutrient availability, energy status, and growth factors. While TORC1 kinase regulation has been examined extensively in tissue culture cells less is known about how TORC1 integrates various environmental and developmental signals within the context of a complex multi-cellular animal. We have characterized multiple components of a new regulator of TORC1 named the Seh1-Associated (SEA) Complex in yeast and the GTPase activating protein (GAP) Activity toward Rags (GATOR) complex in higher eukaryotes. The GATOR complex contains two sub-complexes, GATOR1, which inhibits TORC1 activity in response to amino acid starvation and GATOR2, which opposes the activity of GATOR1. Our studies in Drosophila have begun to elucidate the importance of this new TORC1 regulator in the development and physiology of multi-cellular animals. Importantly, we find that the functions of the GATOR complex in vivo may be considerably more diverse than has been revealed by previous studies.
A conserved nutrient stress pathway regulates meiotic entry during oogenesis.
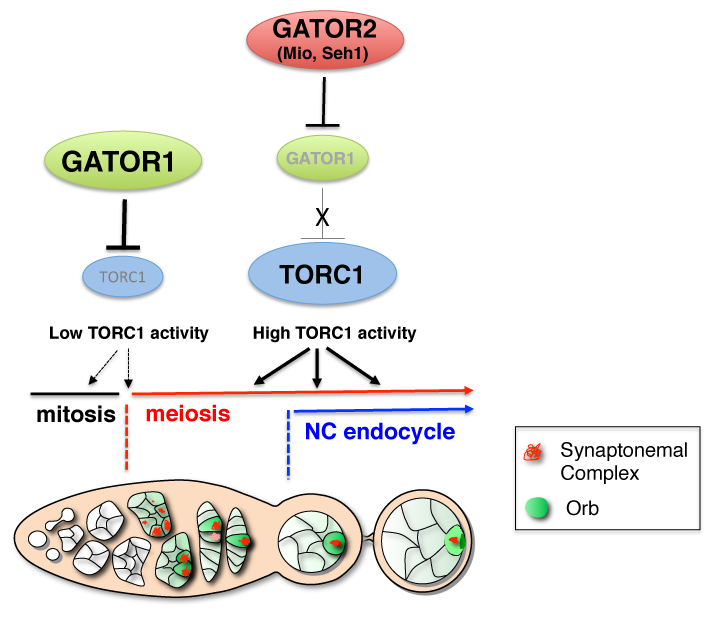
We have defined a role for the GATOR1 complex in promoting the transition from the mitotic cycle to the meiotic cycle during Drosophila oogenesis. In single cell eukaryotes the pathways that monitor nutrient availability are central to initiating the meiotic program and gametogenesis. In Saccharomyces cerevisiae an essential step in the transition to the meiotic cycle is the down-regulation of TORC1 by the GATOR1 complex in response to amino acid starvation. How metabolic inputs influence early meiotic progression and gametogenesis remains poorly understood in metazoans. We have demonstrated that, as is observed in yeast, the GATOR1 complex inhibits TORC1 activity to slow cellular metabolism and drive the mitotic/meiotic transition in developing ovarian cysts of Drosophila. The iml1 gene encodes the catalytic component of the GATOR1 complex. In iml1 germline depletions, ovarian cysts undergo an extra mitotic division prior to meiotic entry. The TORC1 inhibitor Rapamycin can suppress this extra mitotic division. Thus, high TORC1 activity delays the mitotic/meiotic transition. Conversely, mutations in Tor, which encodes the catalytic subunit of the TORC1 complex, result in premature meiotic entry. Taken together, our data indicate that the role of the GATOR1 complex in the regulation of meiotic entry has been conserved from single cell to multicellular animals (Figure 1). These data strongly suggest that catabolic metabolism, triggered by low TORC1 activity, is a conserved feature of early meiosis in many eukaryotes.
Click image to enlarge.
The GATOR1 complex down-regulates TORC1 activity to promote the mitotic/meiotic transition after precisely four mitotic cyst divisions. Low TORC1 activity promotes the mitotic/meiotic transition. Whether the inhibition of TORC1 activity by the GATOR1 complex is required throughout the ovarian cyst divisions or precisely at the mitotic/meiotic juncture is currently undetermined and thus is presented as dashed arrows. After meiotic entry, the Mio and Seh1 components of the GATOR2 complex oppose the activity of GATOR1 complex, thus preventing the constitutive inhibition of TORC1 during later stages of oogenesis. High TORC1 activity is required to drive the nurse cell endoreplication cycle as well as for the maintenance of the meiotic cycle into later stages of oogenesis.
The meiotic regulators Mio and Seh1 are components of the GATOR2 complex.
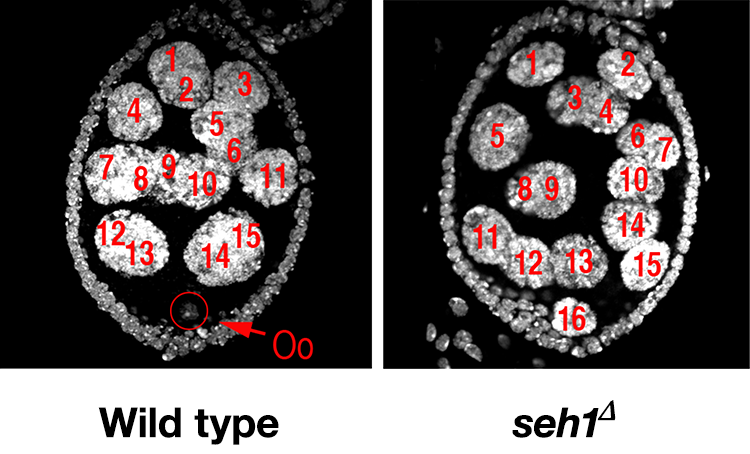
In earlier studies, we identified two genes, missing oocyte (mio) and seh1 that regulate meiotic progression and the maintenance of the oocyte fate. Both mio and seh1 encode components of the GATOR2 complex and are highly conserved from yeast to humans. In mio and seh1 mutants, oocytes enter the meiotic cycle and progress to pachytene. The meiotic cycle, however, is not maintained. Ultimately, a large fraction of mio and seh1 oocytes withdraw from meiosis, enter the endocycle, and become polyploid (Figure 2). Genetic and phenotypic analyses indicate that mio and seh1 act early in oogenesis, before the formation of the synaptonemal complex (SC) and meiotic recombination.
We have shown that the GATOR2 components Mio and Seh1 are required to oppose GATOR1 activity in the female germline to prevent the constitutive inhibition of TORC1 activity and a block to oocyte growth and development. In mio and seh1 mutant ovaries, TORC1 activity is dramatically reduced relative to wild-type controls. Consistent with low TORC1 activity resulting in the activation of catabolic metabolism, egg chambers from well-fed mio and seh1 femalesare filled with large numbers of autolysomes. Thus, in the female germline of mio and seh1 mutants the down-regulation of TORC1 activity and the activation of autophagy occur independent of nutrient status. In our current studies we are exploring the in vivo role of additionally GATOR2 components in the regulation of both TORC1 activity and autophagy.
Click image to enlarge.
 BACK TO TOP
BACK TO TOP