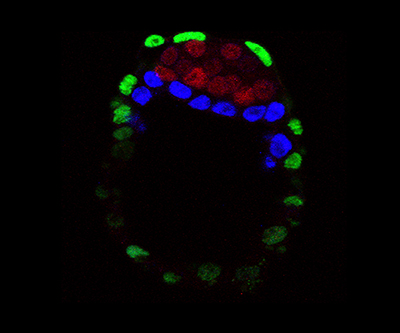
The epiblast layer is labelled red, and the primitive endoderm is blue.
Credit: Rocha Lab, NICHD
During embryonic development, a wide variety of cells, tissues, and organs originate from a handful of progenitor cells. These progenitor cells undergo precise programming to become a specific cell type—a process known as a cell fate or lineage decision. Before a progenitor cell commits to a specific fate or lineage, the cell is considered plastic, meaning it still has the potential to become more than one type of cell.
While much is known about early embryonic development, scientists do not have precise information on how cells maintain their plasticity, which can be lost quickly to give rise to new cell-types. For example, progenitor cells in the early mouse embryo, as early as three days after conception, express transcription factors that predispose them to divergent cell fates later in development—NANOG leads to epiblast cells whereas GATA6 induces primitive endoderm cells. A few hours after this co-expression progenitor cell state, cells decide to restrict their expression to either NANOG or GATA6 by a yet unknown process.
Researchers from the Rocha Lab conducted a series of experiments using mouse embryos to determine how plasticity is maintained in the progenitor state but also rapidly lost. They found that NANOG and GATA6 co-bind the same DNA regulatory elements as a means to maintain plasticity; the dual binding positions a cell to adopt either fate. The co-bound state is quickly followed by eviction and repression of epiblast transcription factors (i.e., NANOG) and a quick remodeling that establishes the primitive endoderm fate through GATA6. Overall, this work offers a new model for understanding early embryonic cell fate decisions.
Learn more about the Genetics and Epigenetics of Development Affinity Group:
https://www.nichd.nih.gov/about/org/dir/affinity-groups/GED.
 BACK TO TOP
BACK TO TOP