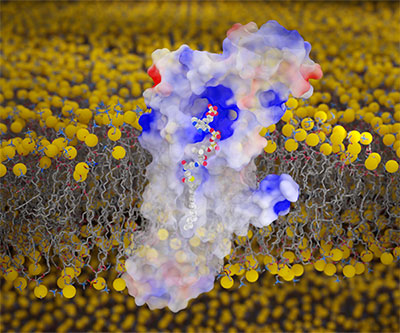
Visualization of fatty acyl CoA bound to membrane-embedded human DHHC20.
Credit: Jeremy Swan. The image was created using UCSF ChimeraX 

Basic science, an important focus for NICHD researchers, enhances understanding of living systems and life processes. This knowledge can later be translated into applications that benefit human health.
This year, scientists funded in part by NICHD generated a synthetic mouse embryo, or embryoid, that closely mirrors natural mouse embryos 8.5 days after fertilization. These embryoids can serve as a model system to investigate factors affecting mammalian embryonic development and disease. Another team of investigators developed a way to assemble gastrointestinal organoids—three-dimensional cell cultures that resemble organs. The researchers coaxed human pluripotent stem cells into components of the three primary cell layers formed in the earliest stages of embryonic development. Combining these three stem-cell-derived cell types led to the creation of complex organoids that will allow scientists to study human organ development in detail.
At the atomic level, scientists at NICHD visualized a key protein modification process called S-acylation, in which fatty acids are attached to certain sites on the protein. S-acylation has been implicated in diseases from cancer to COVID-19, suggesting that strategies to block the process could serve as potential treatments. The NICHD scientists used experimental techniques and computer simulations to gain a detailed understanding of how the enzymes that carry out S-acylation recognize fatty acyl CoA, the molecule from which they transfer a fatty acid to the target protein. This information may help develop methods to block S-acylation.
Another NICHD laboratory identified and characterized two dual-function small RNAs (sRNAs) in the bacterium Escherichia coli. Typically, sRNAs help regulate gene expression—the degree to which genes are turned “on” or “off.” Some also encode a very small protein in addition to this regulatory role. The NICHD researchers unraveled how two such dual-function sRNAs help regulate bacterial growth and metabolism. The findings raise questions about the roles of sRNAs in bacteria, whether derivatives of these tiny proteins could be harnessed to control bacterial cell growth, and how many tiny proteins with regulatory functions remain to be found in higher level eukaryotic cells (i.e., cells with a clearly defined nucleus).
NICHD researchers also study nuclear pores—regulated channels through which molecules enter or exit the cell nucleus—and their related proteins, including RanGAP. In simple eukaryotes like yeast, RanGAP is dispersed throughout the cytoplasm—the liquid interior of the cell—while in more complex organisms, including humans, the protein is targeted to the cytoplasmic side of the nuclear pore. Recent findings suggest that this localization of RanGAP is mostly dispensable for the growth and function of individual cells but may be important for other processes, such as the formation of organs and other larger structures. Identifying these types of regulatory differences can help scientists better understand the circumstances and environments necessary for healthy development.
Studying rare diseases also can improve understanding of healthy development. By evaluating a boy with a rare skeletal disease, NICHD researchers gained insights on how a certain transcription factor—a type of protein that controls gene expression by binding to nearby DNA—is involved in bone formation. Researchers found that the boy harbored a unique mutation in the SP7 transcription factor that altered its binding to specific DNA sequences. The resulting changes in gene expression changes led to abnormal bone formation. The work suggests that other rare genetic disorders could also be caused by mutations that change the DNA-binding specificity of transcription factors and thus affect how cells turn DNA instructions into action.
 BACK TO TOP
BACK TO TOP