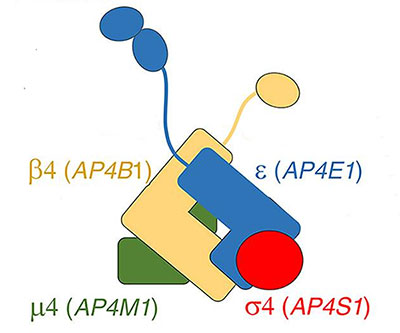
Schematic representation of the AP-4 complex, which comprises the ε, β4, μ4, and σ4 subunits. The names of the genes that provide instructions for each subunit are given in parentheses.
Credit: Bonifacino Lab, NICHD
Adaptor protein 4 (AP-4) is a complex comprising four subunits that helps direct certain proteins to different locations within the cell. Mutations in the genes that provide instructions for any of the subunits—ε, β4, μ4, and σ4—cause a form of hereditary spastic paraplegia called AP-4-deficiency syndrome. Features of the syndrome include weakness and stiffness in the muscles of the lower and upper limbs, intellectual disability and seizures, and brain malformations.
Little is known about the mechanisms by which ε, β4, μ4, and σ4 are assembled to form the AP-4 complex. New work by the Bonifacino Lab shows that alpha- and gamma-adaptin-binding protein (AAGAB) binds to and stabilizes the AP-4 ε and σ4 subunits, promoting assembly of the complex. Cells engineered to lack AAGAB exhibited features similar to those of cells with AP-4 deficiency.
By demonstrating that AP-4 assembly is not spontaneous but rather depends on AAGAB, the findings advance understanding of an adaptor protein complex that is critically involved in development of the central nervous system. They also raise the possibility of increasing AAGAB expression as a potential treatment for certain variants of AP-4-deficiency syndrome.
Learn more about the Cell and Structural Biology Group: https://www.nichd.nih.gov/about/org/dir/affinity-groups/CSB
 BACK TO TOP
BACK TO TOP