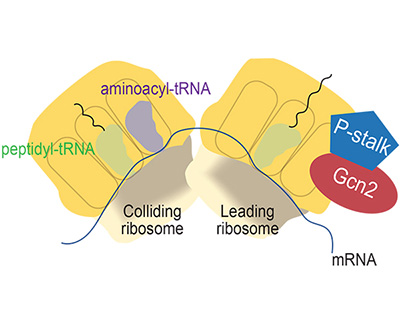
Cartoon schematic of collided ribosomes highlighting the P-stalk and Gcn2
Credit: NICHD/Ritu Gupta, Ph.D.
Cells respond to stressors in various ways. One stress response involves inactivation of the eIF2 enzyme by a process known as phosphorylation. Because eIF2 is essential for initiating translation of virtually all messenger RNAs, impairing eIF2 reduces overall protein synthesis in the cell. It simultaneously stimulates production of the transcriptional activator Gcn4, which reprograms gene expression—the degree to which certain genes are turned on or off.
Gcn2, the protein kinase that is responsible for phosphorylating eIF2, plays a critical role in this stress response. Gcn2 is activated either by a lack of amino acids (the building blocks of proteins) or certain other conditions that stall ribosomes (the cellular machinery responsible for protein synthesis). However, scientists did not know whether the same mechanism of Gcn2 activation applies in both cases.
Recent work from the Hinnebusch Lab indicates that Gcn2 activation has different requirements for the P stalk—a flexible complex of proteins that comprises part of the ribosome—depending on how ribosomes are stalled. The scientists first established that yeast Gcn2 can be activated by three different conditions that elicit ribosome stalling without amino acid starvation. Further experiments revealed that the P1/P2 stalk proteins are essential for Gcn2 activation by starvation-independent ribosome stalling. In contrast, these P stalk components are dispensable for Gcn2 activation by amino acid starvation, suggesting distinct activation pathways for yeast Gcn2.
The findings offer insight into how cells regulate protein synthesis under conditions of stress and highlight the pivotal role of P stalk proteins in this response.
Learn more about the Cell Regulation and Development group: https://www.nichd.nih.gov/about/org/dir/affinity-groups/CRD
 BACK TO TOP
BACK TO TOP