The 2002 BPCA established the initial prioritization process. The process was further refined in 2010 to emphasize the following principles:
- Prioritization must be a well-defined process that includes a systematic approach and clear objectives and outcomes.
- The process must have well-defined objectives and criteria for measuring priority.
- Prioritization must be a dynamic but legitimate and fair process that incorporates transparency, stakeholder input, and strong leadership.
As part of the prioritization process, NICHD annually or biannually solicits nominations from BPCA stakeholders to be considered for priority areas for future consideration. These nominations are reviewed, scored, and tiered by expert volunteers. A sample nomination form is available for review (PDF 69 KB). The BPCA Priority List of Needs in Pediatric Therapeutics is then created, published, and used to inform future clinical research to be considered by the Pediatric Trials Network (PTN) 
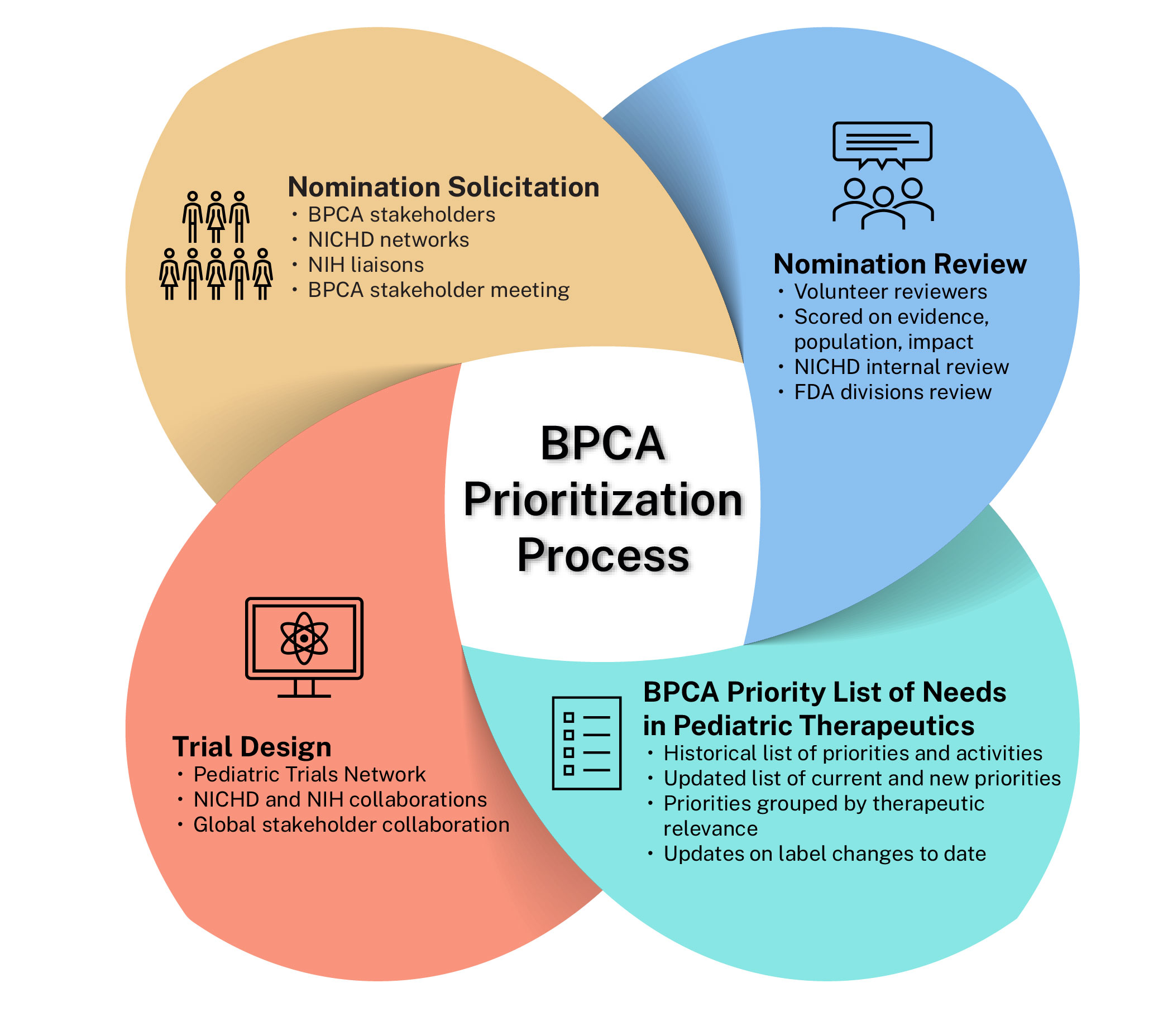
As part of the prioritization process, BPCA convenes working group meetings to address specific therapeutic areas. The working groups have historically included national experts in research and clinical care, along with patient advocacy groups as well as representatives from NICHD and the U.S. Food and Drug Administration. Meeting minutes from all historical BPCA Working Groups are available in the Archives.

 BACK TO TOP
BACK TO TOP