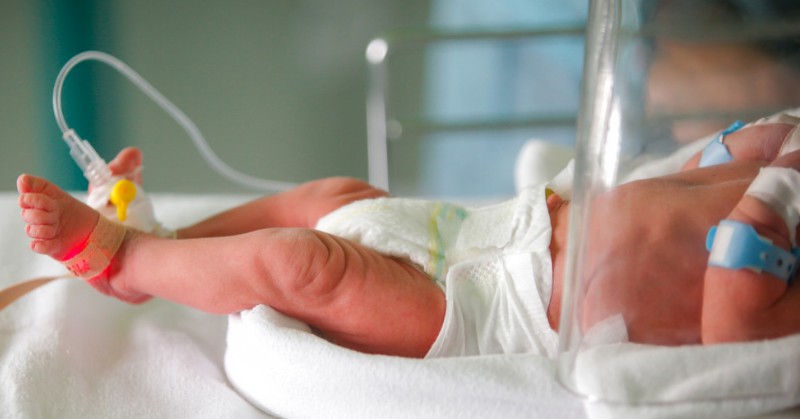
Labels for the antifungal drug fluconazole now include information about its recommended usage and dosage in infants, including those born prematurely. The data that informed these label changes came from research funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), part of the National Institutes of Health.
Many drugs on the market lack specific pediatric safety and dosage guidelines, meaning that health care providers must use these medications “off-label,” or according to best guesses based on adult studies, to treat infants and children. The Best Pharmaceuticals for Children Act (BPCA) program at NICHD supports clinical research through the Pediatric Trials Network (PTN) to provide details about drug safety, efficacy, and dosing in infants and children. Proposed drug label changes based on these data are reviewed and approved by the U.S. Food and Drug Administration (FDA).
Fluconazole, which is available in both oral and injectable forms, is prescribed to prevent and treat various fungal infections. Health care providers often use it to treat infants with candidiasis, an infection caused by Candida yeast. Without treatment, Candida can enter the bloodstream, spread throughout the body, and cause life-threatening complications. Babies born prematurely or at very low birthweight are particularly susceptible to bloodstream infections with Candida. Yet previous fluconazole labels contained extremely limited information about how to use the drug in infants.
The newly revised drug labels incorporate data from several PTN studies and analyses. They include information about how fluconazole is distributed, metabolized, and cleared in newborns and older infants and provide dosing suggestions. The labels also describe data on the safety and efficacy of fluconazole for treatment and prevention of Candida infections in full-term and preterm infants, as well as pediatric patients supported with extracorporeal membrane oxygenation (ECMO) to temporarily replace the function of the heart and lungs as they recover from serious illness or injuries.
“With these label changes, healthcare providers have clear guidance on how to use fluconazole to prevent and treat life-threatening fungal infections in their youngest, most vulnerable, and critically ill patients,” said Perdita Taylor-Zapata, M.D., program lead for NICHD’s BPCA program.
Study data for use of fluconazole in infants with very low or extremely low birthweight are available online. Updated pediatric drug labels are available on the FDA website.

 BACK TO TOP
BACK TO TOP