There is currently an intense effort to develop novel targeted therapies for use in a variety of diseases. There is also increasing interest in approaches to monitor the responses that correlate or even predict the outcome of these illnesses. Early prediction of treatment efficacy can decrease morbidity, and reduce costs. To monitor cancer therapy, the patient has no option other than surgical biopsy or limited radiological evaluation. It is desirable to develop and assess non-invasive, quantitative techniques that can not only monitor structural changes, but can also assess the functional characteristics or metabolic status of the tissue. For anti-angiogenic or pain therapies, factors associated with blood flow are of particular interest.
In these studies, we have developed and are utilizing multiple non-invasive imaging techniques such as near infrared multi-spectral imaging, thermography, and laser Doppler imaging (LDI). The multi-spectral imager is used to reconstruct local variations in skin analyte concentrations oxy- and deoxy-hemoglobin and blood volume. LDI measures the blood velocity of small blood vessels, which generally increases as blood supply demand increases. Combining multi-spectral imaging and LDI allows us to determine changes in blood volume, oxygenation state and blood velocity of the microvasculature and location of the abnormality. The third imaging technique, thermography, provides an integrated thermal signature which combines deep and surface sources and in general can be related to increased blood flow associated with increased metabolic activity.
In combination the use of these three imaging modalities can provide a detailed analysis of tissue function as a predictive tool for the outcome and individualization of therapeutic strategies.
Near Infrared Multi-Spectral Imaging
Light spectroscopic methods are critical to advances in molecular characterization of disease processes. The near infrared multi-spectral imaging system used in our laboratory was designed and built in collaboration with Lawrence Livermore National Laboratory.
Near infrared light penetrates several millimeters into the skin and then reflects back to the CCD camera. Before the photons actually reach the CCD camera, they are filtered so that only a specific wavelength passes through. The images are obtained at wavelengths of 700, 750, 800, 850, 900, and 1000 nm.
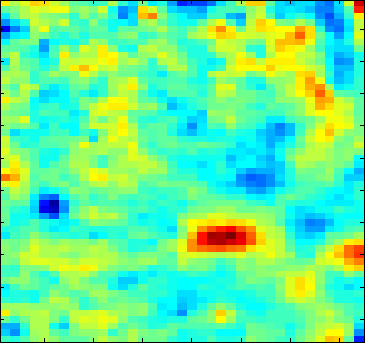
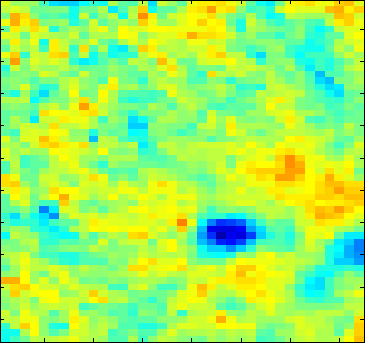
The data from our imager are used to reconstruct local variations in the concentrations of analytes such as oxy- and deoxy-hemoglobin and melanin in the tissue. We have developed a two layered model of the skin in which specific analytes exist in specific layers. The two layers are the epidermis, containing mainly melanin as the absobing chromophore, the second layer is the dermis, containg blood. We assume that the absorption of blood is a function of the volume fraction of oxy- and deoxy-hemoglobin, Voxy and (1-Voxy), respectively.
In practice, we are looking at the intensity at individual pixels given illumination over the entire plane. The six spectral band of our imaging system allows for reconstruction of up to six unknowns.
Thermography
Thermography provides a 2D image of the surface temperature of the skin. Thermography graphically depicts temperature gradients over a given body surface area at a given time. It has been used to study biological thermoregulatory abnormalities that directly or indirectly influence skin temperature. However, skin temperature is only an indirect measure of skin blood flow and the superficial thermal signature of skin is also related to local metabolism. In medical applications, thermal images of human skin contain a large amount of clinical information that can help to detect numerous pathological conditions ranging from cancer to emotional disorders. Advantages of this method are that it is completely non-ionizing, safe, and can be repeated as often as required without exposing the patient to risk. Two different waveband thermal imagers (3-5 and 8-12 micron) are used in our Section.
Laser Doppler Imaging (LDI)
LDI produces a 2D image of tissue blood velocity by scanning a low power solid-state laser beam over the tissue of interest. Low power light from a monochromatic stable laser, such as a 5 mW Helium-Neon laser, incident on tissue, is scattered by moving red blood cells. LDI measures the Doppler shift due to the movement of red blood cells. We use a dual wavelength imager (MoorLDI, Moor Instrument, Inc., UK) for simultaneous scanning at two wavelengths (690 and 780 nm) with spatial resolution of approximately 100 microns to assess blood flow from differing microvascular beds.
 BACK TO TOP
BACK TO TOP