Membrane Mechanics and its Functional Consequences
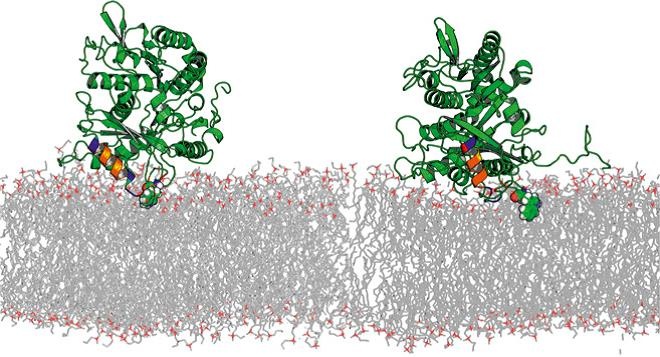
Membrane lipids are no longer regarded as a kind of filler or passive solvent for the membrane protein machinery. It is now well established that lipids play an important role at several levels of cell regulation. This functional involvement naturally explains why cells exquisitely control the lipid composition of their membranes. Still, the mechanisms of membrane-protein interaction and the constraints upon the lipid composition of organelles and cell membranes imposed by Nature are poorly understood. Though the modes by which lipids fulfill their regulatory role are complex and diverse, they can be conditionally divided into specific, i.e., acting through direct chemical interaction with membrane-embedded proteins, and integral, i.e., acting through the changes in membrane physical properties. The latter may be generally referred to as “membrane mechanics” and includes such parameters as membrane stiffness, thickness, bending moduli, and distribution of elastic forces along the lipid molecules. Indeed, when two monolayers of a non-lamellar lipid are brought together to form a planar bilayer membrane, the resulting structure is under elastic stress. Examples from our laboratory demonstrate that this stress can be varied by the choice of the lipid species used for membrane formation and manifests itself in modifying the energetics of hydrophobic inclusions thus influencing protein-lipid interactions and regulating membrane distributions of lipid-soluble drugs, such as volatile anesthetics. Recently, we studied the consequence of the lipid packing stress in binding of peripheral proteins. In both experiments and Molecular Dynamics simulations we found a significantly tighter binding of tubulin to DOPE membranes than DOPC membranes.
Publications:
Jacobs D, Hoogerheide DP, Rovini A, Jiang Z, Lee JC, Rostovtseva TK, and Bezrukov SM (2019) Probing Membrane Association of alpha-Synuclein Domains with VDAC Nanopore Reveals Unexpected Binding Pattern. Sci Rep. 91:4580. https://doi.org/10.1038/s41598-019-40979-8 
M. Weinrich, D.L. Worcester, and S.M. Bezrukov. Lipid nanodomains change ion channel function. Nanoscale, 2017, 9(35):13291–13297.
D.P. Hoogerheide, S.Y. Noskov, D. Jacobs, L. Bergdoll, V. Silin, D. Worcester, J. Abramson, H. Nanda, T.K. Rostovtseva, and S.M. Bezrukov. Structural features and lipid binding domain of tubulin on biomimetic mitochondrial membranes. Proc. Natl. Acad. Sci. USA, 2017, 114(18):E3622–E3631.
P.A. Gurnev, T.C. Roark, H.I. Petrache, A.J. Sodt, and S.M. Bezrukov. Cation-selective channel is regulated by anions according to their Hofmeister ranking. Angewandte Chemie, 2017, 56:3506–3509.
S.M. Rappaport, A.M. Berezhkovskii, J. Zimmerberg, and S.M. Bezrukov. Thermodynamics of inter-leaflet cavitation in lipid bilayer membranes. Physical Review E, 2013, 87(2):022715.
T.K. Rostovtseva, P.A. Gurnev, M.-Y. Chen, S.M. Bezrukov. Membrane lipid composition regulates tubulin interaction with mitochondrial voltage-dependent anion channel. Journal of Biological Chemistry, 2012, 287(35):29589–98.
M. Weinrich, H. Nanda, D.L. Worcester, C.F. Majkrzak, B.B. Maranville, and S.M. Bezrukov. Halothane changes the domain structure of a binary lipid membrane. Langmuir, 2012, 28(10):4723–8.
T.K. Rostovtseva and S.M. Bezrukov. VDAC interaction with tubulin and its physiological implications. Biochimica et Biophysica Acta, 2012, 1818(6):1526–35.
M. Weinrich, T.K. Rostovtseva, and S.M. Bezrukov. Lipid-dependent effects of halothane on gramicidin channel kinetics: A new role for lipid packing stress. Biochemistry, 2009, 48(24):5501–3.
T.K. Rostovtseva and S.M. Bezrukov. VDAC regulation: Role of cytosolic proteins and mitochondrial lipids. Journal of Bioenergetics and Biomembranes, 2008, 40(3):163–70.
T.K. Rostovtseva, H.I. Petrache, N. Kazemi, E. Hassanzadeh, and S.M. Bezrukov. Interfacial polar interactions affect gramicidin channel kinetics. Biophysical Journal, 2008, 94(4):L23–5.
T.K. Rostovtseva, N. Kazemi, M. Weinrich, and S.M. Bezrukov. Voltage gating of VDAC is regulated by non-lamellar lipids of mitochondrial membranes. Journal of Biological Chemistry, 2006, 281(49):37496–506.
V.V. Malev, L.V. Schagina, P.A. Gurnev, J.Y. Takemoto, E.M. Nestorovich, and S.M. Bezrukov. Syringomycin E channel: A lipidic pore stabilized by lipopeptide? Biophysical Journal, 2002, 82(4):1985–94.
S.M. Bezrukov. Functional consequences of lipid packing stress. Current Opinion in Colloid and Interface Science, 2000, 5:237–243.
S.M. Bezrukov, R.P. Rand, I. Vodyanoy, and V.A. Parsegian. Lipid packing stress and polypeptide aggregation: alamethicin channels probed by proton titration of lipid charge. Faraday Discussions, 1998, 111:173–183.
S.L. Keller, S.M. Bezrukov, S.M. Gruner, M.W. Tate, I. Vodyanoy, and V.A. Parsegian. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophysical Journal, 1993, 65(1):23–7.
 BACK TO TOP
BACK TO TOP