Calcium signaling-dependent efficient entry of cationic peptide into cells
Macromolecular drugs, such as polynucleotides, peptide nucleic acids, phosphorodiamidate morpholino oligomers and bioactive peptides, have great potential in the treatment of various diseases. To get to their targets in the cytosol or nucleus, these drugs have to cross the cell membrane that is generally impermeable to most macromolecules. Cationic cell-permeable peptides (CCPPs) are widely considered a promising delivery vehicle for macromolecular drugs and have been successfully used to deliver macromolecular cargo molecules, both in vitro and in vivo. However, we still do not know how the highly charged CCPPs and their cargo cross the cell membranes (PM or endosomal membrane) that are expected to present a non-permeable barrier for the delivery to the cytosol and nucleus. In earlier studies, we and others showed that at 37°C various pathways of endocytosis are involved in CCPPs entry into cells. However, the efficiency of endosomal escape is very low with most of the peptide and associated cargo trapped in endosomes and then degraded. We found that a rapid transfer of cells into cold medium (15°C) in the presence of prototypical CCPP – nona-arginine (R9, 1-2μM) dramatically boosts the efficiency of R9 entry into the cytosol and nucleus detected as nuclear accumulation of the rhodamine-tagged R9. Similarly, efficient entry of CCPPs into cytosol and nucleus can be induced by high concentrations of the peptide (> 10μM, 37°C). We found that interactions of CCPPs with cells activate intracellular signaling cascades that result in significant changes in PM permeability for highly cationic peptides (see Figure). The finding that CCPP entry depends on the cell signaling suggests that manipulation of intracellular Ca2+ level with drugs could be used to promote CCPP entry. Indeed, we found that the nonsteroidal anti-inflammatory drug flufenamic acid both raised Ca2+ level and drastically promoted R9 entry at 37°C, at a 2μM concentration of the peptide. It is still unclear how interactions of CCPPs with the PM induce an increase in intracellular Ca2+ concentration. We suggest that cell surface bound cationic peptide electrostatically modulates the activity of proximal PM cation channels. Indeed, we have previously reported that R9 binding to negatively charged lipids strongly modulates conductance of the gramicidin A channel reconstituted into black lipid membranes. The specifics of Ca2+ signaling pathways that increase PM permeability to CCPPs also awaits further research. Our data indicate that the peptide entry pathways involve PS externalization, as evidenced by the finding that blocking externalized PS by PS-binding protein inhibited both temperature drop- and high concentration-induced R9 entry.
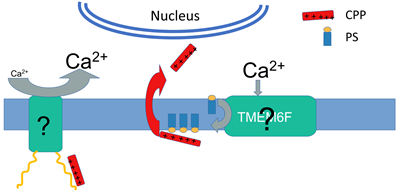
Highly efficient non-endocytic delivery of CCPP into the cell depends on Ca2+ signaling and PS. Upon binding to sulfated proteoglycans on the cell surface, CCPP induces a sustained increase in intracellular Ca2+ concentration and PS exposure, likely mediated by Ca2+-dependent scramblase TMEM16F. Interactions with surface-exposed PS facilitate CCPP entry into the cytosol and nucleus
 BACK TO TOP
BACK TO TOP