Research
Through the advancement of models, methods, and devices that utilize the interaction of light with biological tissue, we strive to develop non-invasive techniques that can help guide therapy and aid in clinical decision making. These techniques are used to perform real-time quantitative measurements of clinically relevant information, including tissue blood flow, oxygen extraction, and body/tissue composition. Our research seeks to move these technologies from “bench to bedside,” where they can be applied to clinical problems, including vascular and metabolic disease.
Aberrations in tissue hemodynamics (i.e., blood flow, oxygenation, and oxygen metabolism) and tissue composition are observed in a wide variety of diseases, including cancer, cardiovascular disease, diabetes, and neurodegenerative disorders. Malignant tumors, for example, lead to the higher blood flow and oxygen consumption needed to fuel rapid cellular growth. In peripheral arterial disease (PAD), long-term buildup of plaque along the artery walls causes reduced perfusion in peripheral circulation. In all such conditions, techniques that can characterize tissue hemodynamics and composition can improve strategies for early diagnosis, screening, and treatment-response monitoring.
There are several clinically accepted ways to assess tissue hemodynamics and composition. Techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) have been used for measuring blood flow through tissue. Additionally, MRI and dual X-ray absorptiometry (DXA) can be used to determine body composition and distinguish between lean and soft tissue. However, these techniques are time- and resource-intensive and can only be accessed in a hospital setting. As a result, patients typically undergo such assessments only after the onset of severe symptoms. As alterations in vascular health occur gradually over time, there is a need for technologies that can assess tissue hemodynamics and composition at the point of care.
Absorption and scattering spectra of lean/fat tissue
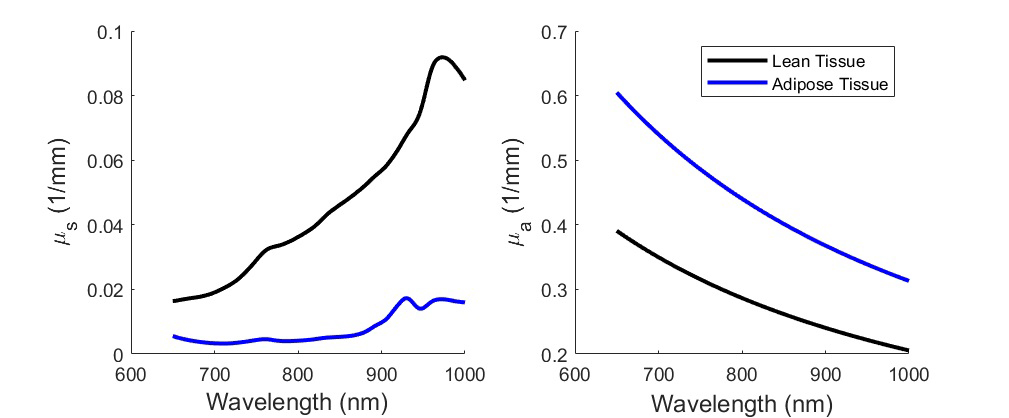
To characterize tissue hemodynamics or composition in portable form factors, near-infrared spectroscopy (NIRS) techniques have emerged as lower-cost and non-invasive alternatives. Techniques such as diffuse optical spectroscopic imaging (DOSI) or spatial frequency domain imaging (SFDI) can quantify the concentration of hemoglobin, water, and bulk lipids. In addition to the compositional information that can be obtained, continuous measurements with these techniques can also assess tissue hemodynamics in terms of the delivery and consumption of oxygen by observing changes in oxy- and deoxy- hemoglobin concentration. While the techniques employ similar principles to measure similar information, they differ in terms of the field of view and depth of tissue interrogated. Another subset of NIRS techniques can quantify blood flow, which is necessary to more accurately characterize tissue metabolic activity. Technologies such as laser speckle imaging (LSI), laser Doppler flowmetry (LDF), and diffuse correlation spectroscopy (DCS) all measure fluctuations in intensity caused by light scattering events from moving particles such as red blood cells, in order to provide quantitative measure of blood flow.
Abdominal DOSI maps of an individual during weight loss
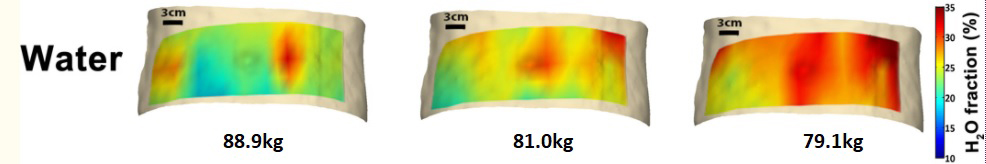
Optical techniques have proven safe and effective in assessing blood flow and tissue oxygenation, but very little is known about the normal range of hemodynamic properties and the degree to which they change as a result of normal physiological variation. It is unclear how much the variations affect standard measurements and whether they contain any novel information. Additionally, tissue composition naturally varies between patients owing to differences in diet and fitness level. Different tissue types have different metabolic activity based on their composition, which will influence overall tissue hemodynamics. The interplay of structure and function necessitates the development and characterization of systems that can characterize both.
There is an urgent need for more powerful and cost-effective wearable and bedside technologies to meet the needs of healthcare providers and patients for personalized medicine and personal health. However, many conventional strategies are insufficient, and there are clear opportunities for new technologies to meet this rapidly growing demand. Tissue optical spectroscopy is widely used in biomedical research and clinical medicine to characterize perfusion, metabolism, and molecular composition and can potentially provide new approaches to meet this challenge. We and others have developed optical methods for detection, diagnosis, and therapeutic guidance in virtually all major areas of medicine such as cancer, cardiovascular disease, diabetes, metabolic disease, and trauma/critical care. In addition, the diffuse photoplethysmographic (PPG) waveform is essential for assessing heart rate in personal health monitors (e.g. Fitbit, Apple Watch, etc.) as well as in medical devices for tissue oximetry and continuous blood pressure monitoring.
Evolution of DOSI systems

While much of this work is conducted using time-independent, continuous-wave (CW) illumination methods that only assess changes in light intensity, a significant and growing body of work shows that time-resolved methods, which employ high-speed modulated (e.g., more than 50 MHz) or picosecond-pulsed sources to measure changes in the temporal dispersion of light, have powerful advantages. Unlike CW techniques, which typically do not account for alterations in optical scattering that can occur during the measurement period and between individuals, time-resolved measurements can quantitatively determine optical path length in centimeter-thick, multiply scattering tissues by measuring either the phase velocity or temporal dispersion of modulated or pulsed sources, respectively. As a result, these time-resolved methods, based on modulated light sources, are used to separate light absorption from scattering and provide substantially greater information content than CW intensity alone. Their quantitative features allow for absolute comparisons between individuals and longitudinal monitoring of subjects with exceptional accuracy and precision over many time points. Unfortunately, current time-resolved methods are prohibitively complex, bulky, and expensive, and generally not suitable for compact wearable devices. With advances in complementary metal-oxide semiconductor (CMOS) and vertical cavity surface emitting laser (VCSEL) technologies, it is now possible to overcome these limitations by creating integrated circuits for time-resolved spectroscopy and imaging. Such state-of the-art, enabling technologies will accelerate the development of new methods and devices with the accuracy, precision, and power of time-resolved techniques, but with the simplicity, low cost, and wearability of CW devices.
The goal of this project is to develop hand-held, easily portable diffuse optical spectroscopy (DOS) systems that perform comparably with previous systems at a fraction of the size and cost. Our primary goal is to develop a DOS system that is driven by a frequency-domain application–specific integrated circuit (fdASIC), which was developed by collaborators at the University of California, Irvine. This will include characterization of the fdASIC performance, design and fabrication of a DOS system driven by the fdASIC, performance characterization and comparison with previously validated DOS systems using known laboratory standards, and in vivo characterization. We will also develop a simpler DOS instrument driven by a commercially available, compact network analyzer in order to streamline the design of the additional optical and electronic components necessary for the fdASIC system.
Obstructive sleep apnea (OSA) is the most common type of sleep apnea, in which the blockage of the airway causes breathing to stop involuntarily for ten seconds or more throughout the night during sleep. When breathing stops, the oxygen level in the blood can drop to harmfully low levels. Pediatric obstructive sleep apnea (POSA) can be especially concerning, with several associated morbidities that can have long-term effects extending into adulthood, including adverse changes in cardiovascular, metabolic, and developmental health. Unfortunately, POSA remains largely underdiagnosed owing to a lack of education about symptoms and limited availability of sleep-medicine physicians. Early diagnosis and treatment are imperative to prevent many of the morbidities.
Polysomnography (PSG), the current standard of care, is expensive, cumbersome, and resource-intensive. At-home sleep apnea tests (HSAT) are less expensive but are equally uncomfortable, prone to user error, and are less effective in children. There is a pressing need for an accurate, robust, unobtrusive alternative for monitoring POSA in the home.
Additionally, the mechanisms of OSA that explain OSA–related outcome measures involving brain health and degeneration are still largely unknown. Approximately 50% of patients with OSA are excessively sleepy during the day, and many develop cardiovascular disease, cerebrovascular disease, and/or cognitive impairment, particularly if untreated. Traditional OSA measures do not predict sleepiness and the apnea-hypopnea index (AHI) and oxygen desaturation index (ODI), measured through standard pulse oximetry, do not typically explain health outcomes, although both hypoxemia and sleep fragmentation are mildly associated.
NIRS is a non-invasive optical technique that can provide direct examination of tissue hemodynamics using near-infrared light in the range of 700 to 1000 nm. Compared with other well-established brain imaging modalities, such as fMRI and PET, the technique has a higher temporal resolution (in order of milliseconds), and provides additional physiological information including heart rate variability and respiration rate. Also, NIRS devices are smaller and can be built into a compact, inexpensive form factor, which is advantageous for ease-of-use and accessibility. NIRS instruments can tolerate subject motion to a greater extent than can fMRI. Such features make the technique ideally suited for studying children, particularly those with problems such as attention-deficit hyperactivity disorder (ADHD), which can make keeping still for long periods of time very challenging. The relationships between NIRS parameters and traditional measurements taken during a sleep study are still largely unknown. NIRS could provide a better prediction of health outcomes and a greater understanding of brain hypoxia during OSA events.
NIRS signals during obstructive apnea events
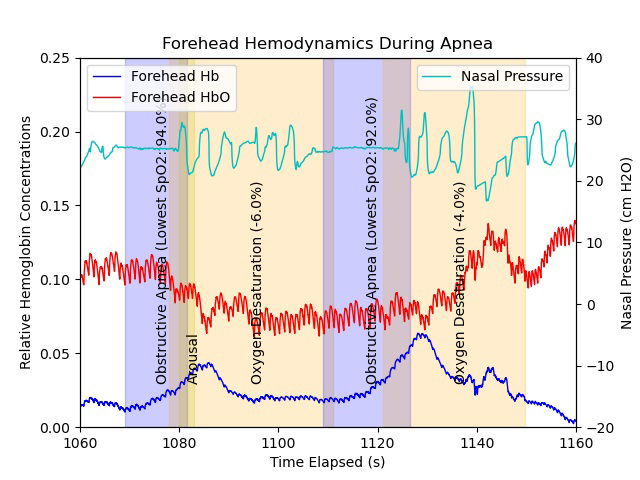
In conjunction with Amir Gandjbakhche as well as Ashura Buckley, we will incorporate NIRS technologies with standard PSG measurement to understand how optical signals change throughout sleep by comparing physiological signals derived from NIRS with data from PSG and how to develop future optical technologies to provide better patient care that can be used in the home setting. We will test the potential of NIRS technology to diagnose POSA and the response of the brain and tissue during an apnea/hypopnea event and investigate these signals before and after treatment.
The emergence of SARS-CoV-2 as a novel coronavirus at the end of 2019 (COVID-19) resulted in a pandemic that has affected millions of people worldwide. Although clinicians and scientists are gaining an understanding of the various presentations and manifestations of COVID-19, limited reports are available on the recovery trajectory and long-term outcomes faced by survivors. With the increasing number of COVID-19 cases being reported in the United States and worldwide, many patients will require follow-up care related to acute respiratory distress syndrome (ARDS), critical illness, and prolonged hospitalization. Those recovering from ARDS and ICU stays typically experience several negative health outcomes, which include physical and mental deficits and reduced life satisfaction and social health. Effective use of aerobic exercise training (AET) as a cardiorespiratory, rehabilitative intervention could have a high impact on patient functional and quality-of-life outcomes.
A portable NIRS platform
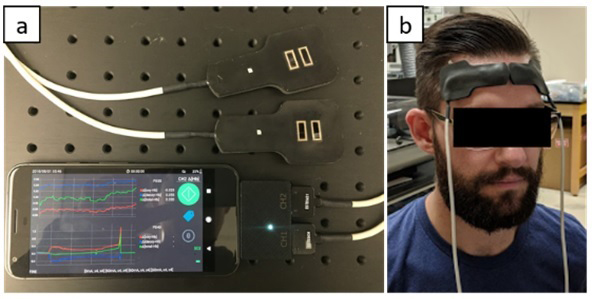
Assessment of vascular hemodynamics is critical for evaluating the impact of any exercise treatment. Additionally, there is growing evidence of the negative impact that COVID-19 can have on the vasculature, leading to endothelial dysfunction and the formation of microthrombi. Current methods to assess vascular health are available in both a clinical setting and as commercially available devices for at-home use. Clinical assessments include standard blood panels in addition to diagnostic modalities such as peripheral arterial tonometry (PAT); such approaches are highly informative but can only be performed in a hospital setting, which limits their accessibility. Consumer-grade devices are significantly more accessible and can even be found in wearable devices such as the Apple Watch or Fitbit. However, the information available to these technologies is limited to standard vital-signs information such as blood pressure, pulse rate, and blood oxygen levels, which is far from a comprehensive assessment of vascular health. Given the chronic nature of the lingering after-effects of COVID-19, the development of technologies that can supply the depth of information comparable to clinical technologies while maintaining the accessibility of consumer-grade devices would be invaluable for characterizing the cardiovascular impact of COVID-19 as well as any prospective treatments.
Hemodynamic changes in the human forearm during an arterial occlusion, as measured by a portable NIRS device
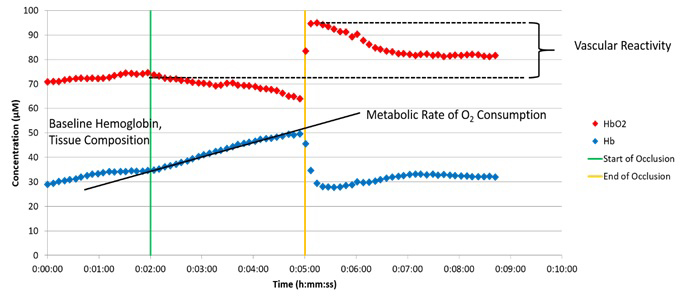
Optical technologies such as NIRS, which non-invasively assess tissue oxygenation, local oxygen consumption, metabolism, and blood flow, have shown great promise as instruments for determining vascular function. Many of these devices are highly portable and could be attractive options for continuous monitoring of vascular function. In a study led by our collaborator Leighton Chan to investigate the impact of AET as a rehabilitative intervention, we will perform a series of optical measurements to characterize the vascular function of patients who have recovered from a COVID-19 infection and are undergoing an aerobic exercise regimen. In collaboration with Manfred Boehm’s group, we will also evaluate the results acquired by these NIRS methods in conjunction with traditional tests of vascular function, including pulse wave analysis, cardio-ankle vascular index, and PAT in patients following COVID-19.
The ongoing COVID-19 pandemic has been a taxing challenge to healthcare systems worldwide. A surge in cases can threaten to overwhelm hospitals' personnel and resources. The wide range and varying severities of COVID-19 symptoms further add to the challenge of managing the disease. While some cases of COVID-19 are mild and require no further intervention, more severe cases can involve major cardiorespiratory symptoms that could require a ventilator. Rapid, point-of-care tests are valuable for identifying patients who are infected with the virus; however, they do not serve as indicators for patient outcomes. A compact method to monitor patient health status and predict patient outcomes could improve our understanding of physiological impact of COVID-19 and alleviate potential strain on hospital resources.
The current standard of care to monitor patient health status revolves around vital-sign parameters such as heart rate, arterial oxygen saturation, respiration rate, and temperature. Some reports have examined personal health devices such as the Apple Watch, Oura Ring, and WHOOP and the continuous health data they all collect, in order to develop models to identify symptomatic patients infected with COVID-19. However, this work still relies on the basic vital sign parameters mentioned above, which only represent a small subset of information on how the body is functioning. Given the growing evidence of the negative impact of COVID-19 on the cardiovascular system, especially the microvasculature, characterization of microvascular health could further improve these predictive models.
NIRS is uniquely positioned to provide distinct information about the delivery and consumption of oxygen in compact form factors. Tissue oxygen saturation, which describes the oxygenation of blood at the tissue and capillary level, is one such metric that can offer valuable insight into microvascular function. In contrast, arterial oxygen saturation, which is normally reported with pulse oximetry, describes the oxygenation of blood coming from the arteries and is therefore an indicator only of oxygen supply. With a sufficiently high sampling rate, the photoplethysmographic (PPG) waveform can also be acquired, which enables NIRS to collect the same vital sign parameters as those collected by commercially available wearables.
We developed a multi-modal optical biosensor capable of real-time, continuous monitoring of tissue oxygenation in addition to vital-sign parameters such as heart rate, respiration rate, and temperature. In order to characterize the performance of our device, we plan to benchmark it to other out-of-the-box wearables. Next, we will measure a cohort of patients with COVID-19 and healthy controls in order to characterize their microvascular health. We believe that including continuous assessment of microvascular health can supplement current patient-monitoring tools, improve understanding of the vascular impact of COVID-19, and aid in the prediction of patient outcomes.
Sickle cell disease (SCD) is a disorder that alters the morphology of red blood cells, which negatively impacts oxygen delivery. As a result of the ‘sickling’ of red blood cells, patients diagnosed with SCD can suffer from impaired oxygen delivery and blood flow, which has many negative downstream effects. Over time, the impact of impaired vascular hemodynamics can lead to multisystem organ complications. While there is no cure other than a bone marrow and blood transplant, there are several treatment options that can reduce symptoms and improve quality of life.
Assessing the efficacy of these treatments as well as the overall health of patients with SCD necessitates characterization of vascular health and hemodynamics. However, current clinical techniques for assessing vascular health require hospital visits, which are both time- and resource-intensive. Point-of-care methods to non-invasively characterize vascular health would be beneficial for optimizing treatments, as well as establishing a more complete assessment of vascular health.
Optical imaging and spectroscopy techniques are uniquely suited to enhance characterizations of vascular health in patients with SCD. Modalities such NIRS, LDF, and LSI can non-invasively quantify tissue oxygenation, blood flow, and hemoglobin concentration in the microvasculature. Additionally, over the last decade research has focused on developing handheld or wearable platforms, which would lessen the need for frequent hospital visits to perform health assessments. We are collaborating with Swee Lay Thein’s group to perform a series of optical measurements on a cohort of patients with SCD in order to characterize their vascular health and hemodynamics and compare their responses to a cohort of healthy volunteers. Additionally, we will work with Thein’s group to perform optical measurements on a cohort of SCD patients as they undergo various treatments, in order to characterize efficacy.
 BACK TO TOP
BACK TO TOP