Report of the Workshop on
Diffusion of ECMO Technology
Extracorporeal Membrane Oxygenation
Sponsored by:
National Institute of Child Health and Human Development, NIH
Office of Medical Applications of Research, NIH
National Institute of Neurological Disorders and Stroke, NIH
National Heart, Lung, and Blood Institute, NIH
Agency for Health Care Policy and Research
Federal Drug Administration
May 31,1990—June 1,1990
Rockville, Maryland
Edited by:
Linda L. Wright, M.D.
Pregnancy and Perinatology Branch
Center for Research for Mothers and Children
National Institute of Child Health and Human Development, NIH
U.S. Department of Health and Human Services
Public Health Service
National Institutes of Health
National Institute of Child Health and Human Development
NIH Publication No. 93-3399
January 1993
Participants
Planning Committee
|
John H. Ferguson, M.D. |
George A. Little, M.D. |
| Elsa Bray (OMAR) | Jennifer Mayfield, M.D. (AHCPR) |
| Charlotte S. Catz, M.D. (NICHD) | George C. Murray, Ph.D. (FDA) |
| Dorothy Gail, Ph.D. (NHLBI) | Philip H. Sheridan, M.D. (NINDS) |
| Gil Hill, M.S. (NICHD) | Linda L. Wright, M.D. (NICHD) |
| Deborah G. Hirtz, M.D. (NINDS) | Sumner Yaffe, M.D. (NICHD) |
Invited Participants
| Robert M. Arensman, M.D. (Chicago) | Arnold D. Kaluzny, Ph.D. (Chapel Hill) |
| Roberta A. Ballard, M.D. (San Francisco) | Martin Keszler, M.D. (Washington) |
| Robert H. Bartlett, M.D. (Ann Arbor) | Lorraine V. Klerman, Ph.D. (New Haven) |
| Mary Anne Berberich, Ph.D. (Bethesda) | Theodore Kolobow, Ph.D. (Bethesda) |
| Alfred Brann, M.D. (Atlanta) | Alfred N. Krauss, M.D. (New York) |
| F. J. Brinley, Jr., M.D., Ph.D. (Bethesda) | Jerold F. Lucey, M.D. (Burlington) |
| L. Joseph Butterfield, M.D. (Denver) | Susan K. McCune, M.D. (Bethesda) |
| J. Devn Cornish, M.D. (San Diego) | Gerald B. Merenstein, M.D. (Denver) |
| Robert A. deLemos, M.D. (San Antonio) | Valerie Mike, Ph.D. (New York) |
| James Dillard, (Rockville) | George C. Murray, Ph.D. (Rockville) |
| Joseph S. Drage, M.D. (Bethesda) | Karin B. Nelson, M.D. (Bethesda) |
| Jonas Ellenberg, Ph.D. (Bethesda) | Michael R. Neuman, M.D., Ph.D. (Cleveland) |
| Michael F. Epstein, M.D. (Boston) | Seymour Perry, M.D. (Washington) |
| Philippe Evrard, M.D. (Belgium/Bethesda) | Charles R. Rosenfeld, M.D. (Dallas) |
| Peggy C. Ferry, M.D. (Tucson) | Alan L. Sandler, D.D.S. (Bethesda) |
| Craig Fleming, M.D. (Hanover) | Rachel Schwartz, M.P.H. (Providence) |
| Roger K. Freeman, M.D. (Long Beach) | P. Pearl O'Rourke, M.D. (Seattle) |
| Lawrence M. Gartner, M.D. (Chicago) | Billie Lou Short, M.D. (Washington) |
| Penny Glass, Ph.D. (Washington) | Hal C. Sox, Jr., M.D. (Hanover) |
| Jan Goddard-Finegold, M.D. (Houston) | Giovanna M. Spinella, M.D. (Bethesda) |
| William H. Hall (OMAR) | Charles Stolar, M.D. (New York) |
| Ann Lennarson Greer, Ph.D. (Milwaukee) | Robert Vannucci, M.D. (Hershey) |
| Ian Gross, M.D. (New Haven) | Richard B. Warnecke, Ph.D. (Chicago) |
| Terry A. Huriburt Ill, M.D. (Hanover) | Alison Wichman, M.D. (Bethesda) |
| L. Stanley James, M.D. (New York) | David D. Wirtschafter, M.D. (Pasadena) |
Contents
Summary with Policy and Research Recommendations
Diffusion of Medical Technology: The Case of ECMO
Ann Lennarson Greer, Ph.D.
Extracorporeal Life Support State-of-the-Art 1990
Robert H. Bartlett, M.D.
Charles Stolar, M.D.
Neonatal Problems Treated with ECMO:
Pathology, Prevention, Alternative Therapies
L. Stanley James, M.D.
Jen-Tien Wung, M.D.
Neurological Outcome After Extracorporeal Membrane Oxygenation
Therapy in the Newborn
Jan Goddard-Fine gold, M.D.
Extracorporeal Membrane Oxygenation Versus
Conventional Medical Therapy for Management of Persistent
Pulmonary Hypertension of the Neonate: A Decision Analysis
Craig Fleming, M.D.
Terry A. Huribut, M.D.
Harold C. Sox, M.D.
Extracorporeal Membrane Oxygenation:
Cost, Organization, and Policy Considerations
Rachel M. Schwartz, MR H.
Katherine K. Willrich, BA.
David E. Gagnon, M.RH.
Summary with Policy and Research
Recommendations
Introduction
This workshop, which was initiated by the National Institute of Child Health and Human Development (NICHD), is the result of the combined efforts of the NICHD, the Office of Medical Applications of Research (OMAR), the National Institute of Neurological Disorders and Stroke (NINDS), the National Heart, Lung, and Blood Institute (NHLBI), the Food and Drug Administration (FDA), and the Agency for Health Care Policy and Research (AHCPR).
A planning committee commissioned background papers which were circulated to the invited participants prior to the meeting. Presentation and discussion of the papers was followed by working groups which met to define policy and research recommendations in the areas of prevention and therapeutic alternatives, identification of populations to benefit from ECMO, research needs, and policy concerns. Recommendations of the working groups were reviewed in plenary session. The resulting Summary with Policy and Research Recommendations is presented below, followed by the commissioned papers.
Extracorporeal Membrane Oxygenation (ECMO) is a new, highly invasive therapy that is being investigated and utilized in newborn infants with cardiorespiratory failure. The apparent increasing use of this technology, especially in new patient populations, as well as concerns about long-term outcome stimulated an invitational forum sponsored by multiple U.S. Government agencies. The specific focus of the forum was diffusion of ECMO technology.
Development and use of this technology in newborns is relatively well defined and documented. The literature includes reports on technical details and clinical application up to and including recent controlled trials. An organization, ELSO, or Extracorporeal Life Support Organization, was formally established in 1989 by investigators and clinicians who previously had been involved in regular meetings and the sharing of information and establishment of guidelines. A patient registry supported by federal and private funds has been maintained.
Current State of Knowledge Concerning Populations to Benefit and Indications for Use
Certain patients with a high risk of morbidity and mortality are appropriate candidates for ECMO when pulmonary function and other studies suggest that mechanical ventilation will be unsuccessful or cause undue harm. ECMO should no longer be considered an extraordinary or rescue therapy for moribund infants over 2 kilograms. Early consultation with ECMO units concerning infants within defined weight and disease categories is encouraged to facilitate timely, safe transfer.
Term and Near Term Newborns
Infants born at greater than 35 weeks gestation with the following disease states should be considered candidates:
Meconium Aspiration Syndrome (MAS) and Persistent Pulmonary Hypertension of the Newborn (PPHN)
A small portion of babies with clinical manifestations of MAS and PPNN may not respond to conventional therapy. Initial stabilization and a trial of conventional therapy should be instituted before consideration of ECMO.
Congenital Diaphragmatic Hernia (CDH)
Mechanical ventilation, general physiologic support, and early surgical repair remain the current standard of care for CDH. ECMO may improve survival in infants with CDH who have continued respiratory failure after repair. Given the increased vulnerability of the congenitally hypoplastic lung to mechanical ventilation, institution of ECMO should be considered earlier in these infants than in infants with PPNN. However, ECMO is not appropriate for infants with severe pulmonary hypoplasia. Delayed repair following stabilization (which may include ECMO) is currently under study and is not at present a preferred treatment.
Sepsis
ECMO is appropriate for a limited number of infants with respiratory failure due to sepsis who do not respond to other therapy. However, studies of children with multiple organ failure (including respiratory) secondary to sepsis, who are treated with ECMO, reveal higher morbidity and mortality compared to other groups treated with ECMO. The indication for ECMO in this group requires further research.
Respiratory Distress Syndrome (RDS)
Apparent RDS in infants of greater than 2 kilograms birthweight appears in a small number of infants and may include other entitles as yet poorly defined. ECMO is appropriate when optimal ventilatory management fails.
Preterm Infants
ECMO is not appropriate for infants under 2 kilograms and 36 weeks gestation except under carefully controlled research protocols.
Postneonatal Pediatric Patients
Respiratory Failure
ECMO is currently being used as rescue therapy for severe respiratory failure in pediatric patients (1 month to 16 years) in a small number of centers. There is an urgent need to define mortality and morbidity risks as well as natural history in this diverse diagnostic group. It should not be assumed that the favorable results of treatment achieved with ECMO with certain term newborn problems can be transferred to those of the older patient. Any use should be considered experimental and should be undertaken with Institutional Review Board (IRB) approval in pediatric intensive care units by experienced pediatric intensivists and ECMO teams with careful data collection and reporting.
Cardiac Support
ECMO is also being used as an adjunct to cardiac surgery in a small number of centers. As in pediatric respiratory failure, these early trials should be done with IRB approval in pediatric intensive care units, by experienced intensivists and ECMO teams, following protocols with careful data collection and reporting.
Patients with Irreversible and Hopeless Conditions
ECMO is a temporizing and not a corrective intervention. Repair and recovery is necessary for patients to benefit. There may be some infants with irreversible organ dysfunction or damage with no hope of correction who may be appropriately excluded from ECMO treatment. Each institution should establish a mechanism of review for such infants.
Prevention and Therapeutic Alternatives
The number of centers that offer ECMO treatment has increased dramatically, however, alternative therapies and prevention have not been adequately explored. There is a serious lack of knowledge regarding epidemiologic factors that influence the requirement for ECMO therapy.
The actual incidence of conditions treated with ECMO, such as PPHN, is not well documented. Furthermore, inter-institutional differences in ECMO utilization are substantial and the relative effectiveness of alternatives versus ECMO is unclear. In the ECMO registry, inborn patients represent only 7 percent of the population receiving ECMO treatment, suggesting that management of outborn patients before they are referred is a major factor determining the requirement for ECMO treatment.
Studies of obstetric and/or neonatal care practices that either prevent or increase the use of ECMO therapy are needed. It is well known that a substantial portion of babies referred for ECMO do not receive the treatment. The ECMO registry might serve as a valuable resource to identify perinatal care practices that influence or determine need. Examples of practices that some feel differ extensively include early recognition and intervention when meconium is present at birth, use of tolazoline, and ventilatory management including the extent of over ventilation.
Particular note should be made of the deficit of information regarding the importance of ventilatory care practices. Retrospective evidence suggests that the mode of ventilation may modify or predispose to a need for subsequent ECMO therapy in infants who develop progressive lung disease. High levels of oxygen alone may result in lung damage; furthermore, the definition of an "acceptable" low level of arterial oxygen (PaO2) varies considerably between institutions. A concept has emerged in recent years that a lower PaO2 than previously accepted might be indicated.
Education and prevention strategies should be developed based upon epidemiologic information about current practices and their impact on the need for ECMO. It is not yet known whether the use of ECMO can be reduced through education of physicians, nurses, and others concerned with the care of critically ill neonates, but the effort should be undertaken.
Based on present knowledge and understanding of the use of ECMO and the infants needing this mode of treatment, the following summary statements regarding prevention and therapies were derived:
- Educational needs of pertinent individuals (physicians, nurses, respiratory therapists) should be determined from practice and epidemiologic studies. Efforts directed toward modifying care practices of the at-risk fetus, the newborn, and the ill newborn should be undertaken. This pertains especially to the physicians responsible for care of the newborn in the delivery room and the physician responsible for management of the infant on a ventilator.
- Continued use and improvement of presently available alternative therapies should be pursued. The use of ECMO for infants who do not meet the presently-accepted criteria for term or near-term infants mentioned above must be considered clinical research and take place only with consideration of alternatives and within appropriately designed trials. Research should continue to determine if there are additional alternatives to ECMO therapy.
Research
The increasing use of ECMO especially in new patient populations creates an urgent need for further research. Clinical studies are needed to determine when ECMO is the most appropriate treatment alternative and what technical improvements are safe and feasible. The short-term and long-term effects of ECMO on the nervous system, pulmonary system, cardiac system, and the blood in all age groups must be further defined. Quality of life as well as specific biologic parameters should be studied.
The Ideal Prospective Randomized Controlled Clinical Trial
The baby at risk for severe neonatal cardiopulmonary problems should be identified before or shortly after birth. The decision to enroll an ill neonate should be made within hours after birth with random assignment to one of several alternative therapeutic interventions. In parallel, the patient should be matched both to a control baby with similar problems of less severity that does not meet study entrance criteria and to a healthy matched control baby. Preintervention parameters should be measured to survey organ dysfunction resulting from the underlying disease process itself. Subsequently, appropriate parameters should be measured during the time of therapeutic intervention. Short-term outcomes (hours to weeks), intermediate outcomes (months), and long-term outcomes (years) should be determined. The idealized times, mentioned above, for the collection of data from patients to be enrolled in a prospective randomized controlled clinical trial are illustrated in Figure 1.
Because of rapidly-changing technological improvements, it is necessary to accrue patients over a limited time period. Multi-center collaboration to ensure adequate patient entry is necessary. A severity and/or prognosis index is needed to ensure balanced risk among the alternative treatment arms.

Figure 1. Idealized time course for a prospective controlled randomized clinical time.
Note: All therapeutic intervention groups and control groops have the same parameters measured before, during, and after the possible intervention period.
Table 1: Parameters and Techniques to Determine Organ Function During Animal Model and clinical Studies
| Animal model Studies | Clinical studies | |
|---|---|---|
| Neurologic | 1. Perfusion/hemodynamics (Doppler) | 1. Perfusion/hemodynamics (Doppler) |
| 2. Electrophysiologic (FEG, EP's) | 2. Electrophysiologic (FEG, EP's) | |
| 3. Non-invasive imaging (NMR, NIRS, US) | 3. Non-invasive imaging (NMR, NIRS, US) | |
| 4. Metabolic (NMR, NIRS) | 4. Metabolic (NMR, NIRS) | |
| 5. Behavior assessment | 5. Neurodevelopmental assessment | |
| 6. Neuropathology | 6. Neuropathology | |
| Pulmonary | 1. Pulmonary vascular resistance control -mediators -anatomy of development mechanics of hyperactivity |
1. ABG |
| 2. Surfactant dynamics | 2. Pulmonary function tests (PFT's) | |
| 3. Surfactant dynamics | ||
| 4. Pulmonary pathology | ||
| 5. Long-term PFT's | ||
| Cardiac | 1. Myocardial oxygen consumption | 1. Ventricular function (echocardiography) |
| 2. Coronary artery flow | 2. NMR | |
| 3. Cardiac pathology | ||
| Blood | 1. Impact of biomaterials on cellular elements and vasoactive substances | 1. Coagulation parameters |
| 2. Impact of circuity on cellular elements | 2. CBC | |
| 3. Vasoactive substances |
Abbreviations:
ABG-aterial blood gases, CBC-complete blood count, EEG-electroencephalography, EPS-evoked potentials, NIRS-near infrared spectroscopy, NMR-nuclear magnetic resonance, PFTs-pulmonary function tests, US-ultrasound.
Assessment of Expanded Clinical Indications
ECMO may eventually prove to be an appropriate therapy for certain premature neonates, but the risks for this population appear greater and the benefits less certain at this time. Any application of ECMO to the premature neonatal population should be limited to strict clinical research protocols.
ECMO following cardiac surgery is currently in use in some centers and requires careful evaluation by clinical research protocols to determine who should be a postsurgical candidate for ECMO and to determine risk versus benefit.
Role of Animal Studies
Animal studies will be necessary to answer certain key questions relevant to clinical studies. Research with animal models to define the pathogenesis and treatment of such pulmonary vascular disorders as persistent pulmonary hypertension could lead to increased prevention and less need for ECMO or its alternatives. A better understanding of the optimal timing for initiation and discontinuation of ECMO may be derived from animal research.
ECMO could possibly be used to simulate the process of in utero hypoxia-ischemia in an animal research model, thereby permitting serial observations and elucidation of pathogenesis.
Specific Studies of Organ Function and Dysfunction
Table 1 lists the key organ systems affected both by the underlying diseases leading to cardiopulmonary failure and by the therapeutic intervention. The critical parameters and techniques used to assess organ function in the clinical situation and in animal models are outlined. With new and promising techniques rapidly becoming available, it will be possible to assess pulmonary, cardiac, blood, and neurologic parameters before, during, and after the ECMO procedure.
Neurologic status is a crucial outcome measure for assessing the long-term functional results of therapeutic intervention. Comprehensive neurologic and developmental assessments by age-appropriate evaluative instruments are needed. Information from neuroimaging, cerebral hemodynamic, neurophysiologic, and neurochemical studies are required before, during, and after the intervention so that outcomes may be related to specific events either preceding or during treatment.
Cardiopulmonary studies require ongoing monitoring of pulmonary function tests, arterial blood gases, and myocardial physiology. Factors controlling pulmonary vascular resistance (systemic mediators, hyperreactivity mechanisms, and changing surfactant dynamics) are of particular interest. Research concerning the effects of intervention on ventricular function and coronary artery flow are also crucial.
Both cellular and plasma blood components can be affected by the ECMO apparatus. Such blood changes (cells, vasoactive substances, and coagulation factors) can have a major impact on systemic physiology.
Studies to Improve ECMO Technology
A number of improvements in ECMO technology are under development. Veno-venous catheterization would be a less invasive technique compared to veno-arterial catheterization. Carotid reanastomosis may prove to be possible. The use of anticoagulative circuits may eliminate the need for systemic heparinization with its accompanying risks. Circuit control and monitoring systems when combined with measurement of critical clinical parameters may increase the precision and delicacy of the intervention. The use of biomaterials as components of the ECMO system would be expected to significantly reduce complications of hypersensitivity or embolization. Careful evaluation of the immediate efficacy and safety, as well as the long-term outcome of these various modifications will be necessary.
Studies to Improve Alternative Therapies
Alternative treatments for cardiopulmonary failure in the neonate are currently being explored. These include the use of surfactant, high-frequency ventilation, negative-pressure ventilation, liquid ventilation, and nitric oxide. A spectrum of "conservative" or "conventional" ventilatory assistance interventions, which are either already in use or are being developed, require controlled trials to determine their relative effectiveness.
Outcome Analysis (Follow-up Studies)
All studies require rigorous delineation of outcome analysis. The short-term outcomes include death, neurologic function (including EEG, neuroimaging, and clinical parameters), and cardiorespiratory function as defined by physiologic parameters. The long-term outcomes include death, health status (disease, disability, morbidity), and both global and system-specific functional status (psychomotor development, quality of life, and social adaptive functioning). Long-term outcomes also should be analyzed with respect to total health care utilization and cost.
The ultimate goal would be to analyze specific factors present before or during the ECMO procedure with regard to possible relationship to specific functional deficits detected at follow-up. It is essential to follow children long enough (school age or beyond) to assess motor performance and higher critical functions, including right versus left hemisphere cognitive processes. Contacts between physicians and parents established at the beginning of ECMO therapy should be maintained to facilitate study.
Policy Considerations Influencing the Diffusion of ECMO
ECMO is a relatively new technology, so it is not surprising that few, if any, policies currently influence its use at existing centers or its spread to other facilities. Even technologies with longer histories have few formal policies governing their use. Decisions about who will perform medical and surgical procedures and where they will be performed are often under the control of hospitals and other health care facilities, as well as being strongly influenced by the organizations that pay for such procedures. Professional organizations, accrediting bodies, the Federal Food and Drug Administration, and state regulatory agencies also establish policies.
To make ECMO available to those infants who would benefit from it, and in the most cost effective manner, the development of three types of policies is recommended:
- Those that would promote effective and efficient use of existing ECMO centers and avoid inefficient and expensive proliferation.
- Those that would monitor the quanity and quality of ECMO treatment.
- Those that would encourage long-term studies.
To promote effective and efficient use of existing ECMO centers and avoid inefficient and expensive proliferation, the following should be implemented:
- Planning at the state and regional levels. This should include obtaining epidemiologic information that can be used to forecast the need for ECMO in various geographic areas.
- Development of guidelines for ECMO centers. Such guidelines should include staffing needs, minimum number of cases per year, acceptance criteria, data to be collected and submitted to the registry, quality criteria, and other aspects of care. (For the present, such guidelines should be limited to the use of ECMO with neonates.) Expansion of the technology for the treatment of pediatric or adult patients would necessitate the development of additional guidelines. Several organizations should be involved in the development of additional guidelines. The Extracorporeal Life Support Organization (ELSO) has already developed guidelines and the Committee on the Fetus and Newborn of the AMerican Academy of Pediatrics (AAP) has issued "Recommendations on ECMO" (Pediatrics 85:618-619, 1990.) These groups should be encouraged to remain active in guideline development. The Agency for Health Care Policy and Research (AHCPR) now has a mandate to develop practice guidelines; although its work to date has been primarily in relation to practices supported by Medicare, it should be expanding to tother fields in the near future. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) should be asked to become involved in accreditation of ECMO centers (see next bulleted section).
- Development of an accreditation procedure. ECMO centers should be accredited, or licensed, or designated. Models for such accreditation are those currently used by the 10 American College of Surgeons (ACS) for Cancer and Trauma Centers. The JCAHOmight be asked to form and ad hoc ECMO Accreditation Task Force, which shouldinclude representatives from groups such as ELSO, AAP and ACS. Other groups that might be involved in the process are federal and state rate-setting agencies and state maternal and child health agencies because of their concern for children with special health care needs.
- Encouragement of the developement of hospital "strategic alliances." Hospitals can work together to promote efficient utilization of existing centers through regional cooperation. Alliances have the potential to stabilize referrals, secure economy of scale, and promote continuing education of staff. This model of collaboration already exists in the Community Clinical Oncology Program.
- Development of funding policies that encourage use of existing ECMO centers and discourage inappropriate proliferation. Medicaid and other third-party payers should provide reimbursement for ECMO treatment outside of the state in which the infant resides and for transportation to and from ECMO regional centers.
To monitor the quality and quantity of ECMO treatment, the following recommendations concerning data and case registration are made:
- Continuation and improvement of the existing ELSO case registry-The case registry should be used as a management and research tool in order to determine efficacy and effectiveness. It should be able to indicate quality problems such as institutions that treat too few infants or have high failure rates. It should be used to help defelop long-term outcome studies.
- Formation of an advisory board—This board should develop a list of data elements and undertake other relevant tasks, such as the development of procedures and incentives for establishing and maintaining data quality.
- Development of a permanent funding base— Funding of the registry should be the ultimate responsibility of participating ECMO centers.
- The Federal Government and foundations have assisted with initial efforts and should continue in the short term.
- Participation in the registry should be required for accreditation.
- The registry should be used to monitor the relevance of guidelines.
Diffusion of Medical Technology:
The Case of ECMO
Ann Lennarson Greer, Ph.D.
University of Wisconsin—Milwaukee
Abstract
This overview distills accepted diffusion theory and the author's ongoing research to outline factors and dynamics which come into play around high-cost, high-risk, highly-complex technologies like Extracorporeal Membrane Oxygenation (ECMO). Three themes are developed. The first is the need to understand adoption of new technology as a social process wherein a consensus for change develops among local medical colleagues who communicate directly with one another in a situation of common circumstances, pressures, and experience. The second concerns the fact of medical technologies such as ECMO diffusing into practice even as they continue to change and develop. With such dynamic technologies, the state-of-the-art is as much a consequence of practice as of science. The third theme concerns the fact that hospital technology adoptions engage multiple interests, decision-makers, and criteria for adoption. The assessments of doctors are combined with those of businessmen and organizational planners in a dynamic local process which drives adoption and use. There is a brief discussion of the difficulties of using guidelines to affect this process.
The Diffusion of Dynamic Technology
The diffusion of an innovation is a highly social process. The spread of even a simple technology, a new drug for example, is characterized by many interpersonal contacts and differentiated social roles—change agents, idea champions, early and late adopters, and opinion leaders.1'2 Except for quacks and cranks and geniuses recognizable only after the fact, innovation is adopted not by isolated individuals alone with their journals but by networks of people who adopt more or less as a body, following a change in the local consensus about what one should do and be seen doing. Initial awareness of an innovation may come through reading but one-on-one activities—observation, trial, and social reinforcement—are usually necessary to adoption. The spread of a technical innovation is not so different from the spread of a new dance step. Most want to be neither first nor last, perform badly, or look like a fool.
The desire for consensus is most understandable. Research findings and their application are rarely clear-cut. In our studies, doctors have shown themselves to be skeptical of their professional literature. (It could easily lead you to foolishness.) They cite the over-optimism of scientists and developers in interpreting early findings, the frequent inconsistency of published results, and the coming and going of touted procedures. In addition to confusion over results actually achieved at research sites, local physicians have many uncertainties regarding their personal ability and the ability of their colleagues to achieve benefits in their practices. They fault the medical literature for omitting details of application and report skepticism regarding the application of findings to their own patients and institutions. Personal contacts made at professional meetings or instructional seminars permit resolution of some difficulties but uncertainties ordinarily slow adoption until time and local opinion support (or may even seem to demand) behavioral change.3'4'5
In shaping the local consensus, opinion leaders are key actors. Found in all groups, opinion leaders are respected by their local colleagues both for their competence and for their commitment to the welfare of the group. Colleagues trust that they will know what a new thing is about, what it means to the group, and that they will not steer associates wrong. Their acceptance of an innovation portends adoption by other members of their social network.
Assessing new technology in its relation to local circumstances may be difficult. Allen contrasts the acceptance of scientific findings with the introduction of new technology. Scientists share a universal language and common criteria for judgement. Technology is local, defined by local goals, problems, people, facilities, equipment, systems, and values.6 Appraising the implications of a new technology may then presuppose its prior local demonstration. When this is the case, successful introduction requires a local innovator, a technical person who is able to adapt the technology to the specific situation. This innovator's activities may, in turn, affect the technology's ongoing development, creating a dynamic interaction between concept and practice.
The difficulties of application compound when the technology is itself a complex mix of human as well as technical components. The potential for interpersonal problems can lead to another role which is associated with successful implementations. This is the advocate, or idea champion. Internal to the adopting group or organization, this champion becomes committed to an innovation's acceptance. The idea champion keeps the idea alive through the innumerable organizational, financial, and political hazards which beset any undertaking that has the potential to absorb scarce resources or alter personal relationships. Both the technical innovator and the idea champion must be in a position to devote time to the tasks of reconciling technology and local circumstance.
Rogers has used the term "re-invention," to indicate the degree to which an innovation is changed by adopters in the process of adopton and implementation.2 Not only may the tool be changed, the technology may be used by different personnel, combined with other technologies in new ways, or used for purposes other than those originally intended. In this process of trial and modification, ambiguities are removed and possibilities are revealed or restricted. In the case of dynamic technologies, what diffuses is not so much a prepackaged product as a concept that is specified as it is applied.
ECMO does not appear to be an exception. ECMO offers a high tech, high cost, labor and equipment-intensive rescue for newborns suffering from intractable respiratory failure. External cardiopulmonary support is provided to the infant by a modified heart-lung machine so that the damaged lung may rest. It is a technically high-risk procedure which ordinarily includes ligation of the right carotid artery and jugular vein through which the support system has been connected. Claims of remarkable results by developers have generated controversy in the literature over 1) the design of evaluations which have, for the most part, lacked a true control group7 and 2) the baseline predictions of mortality against which the survival of ECMO babies is so startling. Some writers have suggested that the high mortality associated with pre-ECMO management of candidate babies may have been iatrogenic.8,9
The technology itself is changing rapidly. Even as the University of Michigan developers of ECMO are proposing a new means of cannulation to avoid permanent ligation of the carotid artery, other surgeons in other locations are reconstructing the artery.10, 11 Independent teams are varying other components also, such as the time during which infants are maintained on ECMO.11 Conditions for expanding the indications for ECMO are being discussed as are possible new uses. Donn reports optimism that new technical developments will open the procedure to premature infants and prove useful in preserving transplant organs or as "a temporizing device for cardiac surgery."10 He reports that the development of expertise in ECMO centers has made possible the application of technologies other than that which organized the teams. Some ECMO programs are able to treat up to one-half the babies referred for ECMO with conventional methods.10 ECMO team members are drawn from several specialties and tasks are performed by members of more than one specialty with the key supervisor, for example, being sometimes a pediatric intensivist, sometimes a neonatologist, and sometimes a pediatric cardiac surgeon. This might suggest an early division of opinion as to whether childrens' hospitals or neonatal intensive care units (NICUs) attached to large obstetric units are to be preferred locations for ECMO technology. Finally, experience with a wide variety of technologies from ultrasound to lasers shows how quickly new technologies jump the boundaries of their originating specialties when medical tinkerers see them in action.
With dynamic medical technologies, the experience of early (and not so early) adopters may be considered part of a technology's development. These technologies develop as they diffuse with adopters' activities complementing or diverging from the work of the initial developers, whose work may also be ongoing. This process of adaptation may seem to develop or to betray the original innovation. It leads to inconsistencies in results and in the published literature and to variations in local practice. Different versions in different hands provide different localities with differing experiences which may lead to inappropriate curtailment of use in one community and to excessive optimism in another. While highly significant in affecting local assessment, diverse experiences may or not make it to the published literature and may or may not be considered to contribute. Patients will have been selected differently, procedures will have been performed differently, outcome measures will be inconsistent, and few will have adequate controls.
Multiple Vantage Points for Adoption Decisions
If it is difficult to develop useful guidelines for medical doctors considering adoption or use of dynamic medical technologies, it is much more difficult to guide adoption when a variety of non-medical concerns and pressures come into play as they do in the operation of contemporary hospitals.12'13 While it is tempting to focus on medical criteria, other decision criteria may constrain or impel adoption and use. Indeed, our data indicates that medical doctors are less involved in hospital decisions than a decade ago and medical criteria are less influential.14
Three types of assessments affect the adoption of hospital-based technologies such as ECMO. These are summarized in Table 1. Medical criteria are applied by doctors, both those who will apply ECMO and those who will refer patients for it. But these decision-makers confront two types of organizational decision-makers who draw their decision criteria from the fiscal/ managerial and strategic planning domains.
Medical Assessments Affecting Adoption by Physician Users
In many studies certain attributes of innovations have been observed to affect physician adoption of a new technology. Among those commonly identified are: relative advantage over previous methods, risk, compatibility with adopters' values, understandability, and complexity of use.2,3 Each of these attributes is actually a subjective judgement made by a potential adopter who perceives risk and complexity in relation to personal skill and experience, advantage in relation to failure or success with alternative methods, and complexity in relation to professional backup and organizational capabilities. Given the many questions that are likely to be unanswered in the literature and the difficulty of integrating change into one's practice, the most common result is to "wait and see"—where the findings of the literature will settle, what models will become available, how one's local colleague's will respond, where and when the local consensus will gel.
ECMO belongs to a small category of desperation technologies that may attract unusually positive assessment since they offer hope where there was no hope. Relative advantage is calculated against death; calculations of risk may also seem niggling. The absence of scientific data on long-term outcomes leaves physicians without the ability to include in their assessment important aspects of risk, benefit, and compatibility with values. In the absence of comprehensive outcome data, application is likely to emphasize (1) short-term goals, (2) theoretical plausibility of benefit, and (3) one's level of satisfaction with alternative treatments.
Despite the compelling thrust toward diffusion provided by desperation technologies, adoption remains problematic. Most physicians are very concerned about their ability to perform morally and proficiently within professional guidelines. Application of a technology may involve pain, expense, and anguish for patient and family. Two attributes of innovations that are commonly associated with adoption are firsthand observability of benefits and potential for hands-on trial. Opportunities for observation and training may be initially limited to those offered by developers. Accordingly, they may vary, although many innovators are also energetic educators who open their units to interested colleagues to observe and learn. Well into the diffusion of surgical procedures, it appears that existing sites of activity are the most important dispensers of education and trial.
The personal experience acquired at such sites is often a motivator to further action but it may also highlight dangers, such as a high complication rate in the learning stage, or difficulties, such as those encountered in mounting a new program. Manufacturers have long been attuned to the need to hand carry reassuring information to potential but skeptical users of their products, and to reduce those barriers over which they have some control, such as providing staff training, technical backup, or a period of equipment trial.
Table 1: Types of Hospital Technology Assessments
| Dimension | Medical-individualistic | Fiscal-managerial | Strategic-institutional |
|---|---|---|---|
| Prominent actors | Physicians, primarily specialists | Chief executives, fiscal officers, employed department heads, hospital-based physicians | Governing boards and chief executives |
| Espoused ideologies and values | Maximizing patients welfare, avoiding risk | Rationality, predictability, financial viability, "profitability" | Formulating and realizing viable institutional missions, securing and protecting future options. |
| Bases of influence | Professional training, clinical experiences, colleagues' respect | Reputation for financial, computational, and marketing ability | Authority, credibility, and charisma |
| Typical structuring of decisions | Professional collegia, medical staff committees | Embodied in standardized procedures and analytical programs | Long-range planning and policymaking bodies |
| Typical decision processes | Assessing clinical effects consensually, tempered by norms of professional deference | Conducting cost-benefit analyses, discounted cash flows, etc. | Forecasting impending threats and opportunities, constructing alternative scenarios |
| Information gathering and utilization | Information obtained, personal experience, professional media, and short courses of developers/advocates | Systematic search, quantitative evaluation | Information gleaned from diffuse sources combined and extrapolated holistically |
Medical Assessments Affecting Decisions of Referring Physicians
Physicians who refer patients to specialized facilities also grapple with assessment of benefit and risk but from a somewhat different perspective. These physicians are less likely to be abreast of specific findings concerning the procedure and do not expect to make a final determination on appropriate application. They are likely to become aware of new modalities such as ECMO through a personal contact with a person who is familiar with the technique. Physicians affiliated with the hospital offering a new treatment are likely to produce the first referrals. Referrals from neighboring institutions are likely to be next. Most often these will be stimulated by contacts that referring physicians have with the local innovators or from contacts that these physicians have with other physicians who are aware of the option—rotating residents, circuit-riding colleagues who practice at multiple hospitals, or newly affiliated physicians. This process of information diffusion may be speeded by continuing education or marketing activities of the sponsors or the sponsoring hospital, local professional associations, manufacturers, or medical schools.
Referring physicians will weigh problems of patient transfer against possible benefit. Referral for a life-saving technique is likely to be appealing, although enthusiasm for referral is often tempered by the physician's general dislike of transporting patients to another institution. They dislike the hazards and inconvenience associated with referral and may perceive the potential to lose a patient to physicians at the referral center. The more referrals from a specific hospital to a referral center expand, the more physicians at the former seem willing to consider local development of the program. This possibility seems reduced to the extent that ECMO is a self-limiting procedure offered at specialized children's hospitals, although it is possible to imagine that neonatologists at a non-ECMO center might fear loss of referrals to a NICU having the ECMO backup. As a technology such as ECMO is refined, indications are expanded and additional uses are contemplated (e.g., ECMO as a backup for surgery vexed by respiratory failure), the arguments for additional programs may grow.
Fiscal and Managerial Considerations Affecting Adoption
Current payment strategies that fix the amount that will be paid for procedures, either annual patient care or specific diagnoses, were intended to make adoption of expensive but seldom-used technologies unattractive because they are unprofitable. The acceleration of the hospital "arms race" that has accompanied implementation of competitive policies suggest that they do not work in the way intended.15
In response to the new payment policies, the ranks of financial, marketing, and quality control workers in hospitals have swollen. These specialists provide expert analyses of the fiscal and managerial aspects of the organization that affect the bottom line, including technology. These staffs work with such aspects of the organization as management of volume, billing, patient mix, contracts for services, and position in the capital market. They suggest means to maximize and regularize income and control expenditures in ways that accommodate the fixed payments that hospitals have agreed to accept. Toward this end, they combine cost-control and marketing strategies, trying both to hold down costs and increase patient revenues. Such financial calculations are supposed to favor economies of scale and the avoidance of activities where insufficient volume produces high "per unit" costs. However, for each strategy of curtailment there appear to be alternatives, such as increasing volume and providing as many services as possible internally. It has been suggested that administrators of hospitals containing ECMO units may encourage an expansion of indications for its use in the interest of achieving financial solvency for the unit.10 Similarly, in my area, HMO's have reportedly questioned the need to refer babies out of a prepaid neonatal center to an ECMO center where additional costs must be paid.
In advising the development of new programs such as ECMO, fiscal and managerial staff are asked to calculate the period required for "payback," given start-up costs, payments, and projected volume. However, an unfavorable calculation is more likely to restrain low visibility expenditures—routine equipment purchases, staffing, or research—than those with high visibility or potential visibility. Even under rate review, hospitals' cost-reducing strategies in the area of technology were directed primarily to second pieces of equipment and replacement of equipment, not to new introductions.16 Under market competition, there is increased pressure to direct funds to areas that enhance the hospital's visibility, market share, or potential to tap new markets.
Strategic Planning Considerations Affecting Assessment
In recent years, the importance of strategic plans to hospital management has steadily increased.17 The competitive pressures of policies introduced since 1984 have accented the importance of targeting development to achieve desired position in the marketplace. "Markets" may be diagnoses that are favorably reimbursed under DRG's, or populations that are well-insured, and one market may be perceived as connected to another. Financial and managerial calculations are advisory to strategic planning, which focuses heavily on the image that the hospital wishes to convey. How costly will it be to convey the desired image of a hospital as better or at least as good as competitors? What is the cost of increasing market share, capturing new markets, or securing future options? Money may have to be saved somewhere in the hospital, but it will not be in areas targeted as strategically important, given concern with image, payment, demographics, or interrelationships with other services. In these cases, the issue is not the balance sheet, but survival viewed strategically.
Strategic planning may dictate the development of ECMO programs, irrespective of potential financial losses. In my area, cardiac surgery offers a prior example. Hospitals have continued to introduce new programs in spite of a dense supply. Administrators argue that heart surgery is a necessary backup to angioplasty and therefore to cardiology, and because "ambulances will bypass a hospital that does not have heart surgery." They closely watch outward referrals with dismay, often envisioning a domino effect. For similar reasons, it has been difficult to sustain the regionalization that once characterized neonatal intensive care. Obstetrical care is dispersed among hospitals which, if it can be avoided, do not want to refer mothers to regional centers from which they may not return. As one neonatologist told us:
"I mean the perinatologist [at a regional center] has to work hard to tell the [referred] mother 'I've taken care of you during this high risk delivery. Now you go back to your regular obstetrician in the suburbs. Okay?' The hospital in the suburbs has to work hard to have itself identified as being still the place that is going to provide medical care for that family and not have the mother say, 'God, when I have been really sick and I had that real bad baby and I went into the hospital in the middle of the city at the medical center, I had such great care. God, if anybody in my family ever gets sick again, we're going right back there."
Hospital administrators routinely cite outward referrals in their arguments for the adoption of a new technology or service. ECMO seems susceptible to such pressures, especially since the existence of superior neonatal intensive care is often advertised as a reason that prospective mothers should choose a hospital's obstetrical unit. Obstetrical care, in turn, is viewed as the entry point for families to life-long hospital care and an important element in the full-service package sought by HMO's.
When are medical, fiscal/managerial or strategic criteria important? How do the decision-makers who employ them exert their influence? What concordance among decision-makers is necessary for a chosen policy to be realized? To inform answers to these questions, the next section will be devoted to developing a brief prototype of adoption dynamics. This simplified representation contains characteristic elements that we have encountered repeatedly across diverse technologies.
Prototype Adoption
Although information on a new technology may be appearing and being noted by local physician communities, active local consideration awaits the emergence of a local medical innovator. This first adopter affiliates with a developing technology, perhaps early enough to be a major contributor, or somewhat later in its evolution. This local innovator is linked from past training or experience to national or regional pioneers of the technique, or now becomes so linked. When the demands of the technology require large expense or organizational adjustment, the innovator is successful to the extent that an idea champion is engaged. This is frequently a hospital administrator who seizes upon the idea of securing a new market or positioning the hospital for future gains. The hospital board is persuaded of the importance of the new development. The program is organized. The innovator addresses technical problems and trains colleagues and staff, while the administrator addresses problems of finance, recruitment, turf, and securing a referral base for the procedure
The existence of the program alters the local environment. Potential new adopters and referrals surface as the new activity (1) generates visible applicable clinical evidence; (2) opens opportunities for training of both residents and existing specialists; (3) attracts attention of media and patients, thus generating publicity and patient inquiries; (4) focuses attention on merits of the development (wise or unwise, wisely located or misplaced); (5) spotlights the behavior and referrals of physicians in relevant specialties (Do they perform? Refer locally? Refer to more distant sites?); and (6) provides ammunition to equipment or other salesmen who will manipulate the appearance of consensus and rapid diffusion in the hopes of expanding utilization. All of this speeds the need of pertinent specialists to develop a professional opinion, potentially speeding the development of a consensus for change.
The larger community of physicians is affected by (1) a surge of information following local adoption (brochures, lectures, demonstrations, referral solicitations) from the provider hospital, manufacturers, newspaper reports, continuing medical education, and gossip; (2) circulating individuals who are aware of the new activity; and (3) patient inquiries stemming from newspaper stories or contacts with early patients or their families. This turbulence in the local arena demands a response and raise a suspicion on the part of physicians and hospitals that patient choices of physician and hospital may be affected by this.
Hospital administrators are aroused as the lead hospital markets the new technology to potential patients as a mark of superiority and to HMO's as a reason to become a member health care provider. Administrators of rival hospitals perceive the new technology as a threat to their own high technology image, to viability of their own competing units, and possibly thus to the whole of the hospital. They begin to monitor outward referrals, inquiries from patients and HMO's, outward transfer of prospectively budgeted funds, image benefit to other institutions, and the overall effect of the development on competitive relationships with rival hospitals. They consider their own position in terms of advantages to be gained from offering the technology and the chances of capturing existing or future market share. If the development is viewed as a serious threat or serious opportunity, hospital executives may encourage physicians interested in performing the procedure or begin recruitment of physicians with needed skills and interest.
The Role of Guidelines
Information circulates as this drama plays, but seems not to be master of the forces at work. My own studies of the diffusion of innovation began in 1975, when open heart surgery could be observed in its still early stages in my city of Milwaukee. In one of the first half dozen interviews of what now approaches 800, a local heart surgeon, Dr. Derwood Lepley, pressed into my hands the 1972 report of the Committee on Coronary Artery Surgery of the Intersocietal Commission for Heart Disease Resources. The Intersocietal Commission had as its sponsors virtually every professional society and government institute bearing any relation to heart, surgery, or hospitals. The report was entitled "Optimal Resources for Coronary Artery Surgery."
The committee had been chaired by Dr. Lepley. The report specified the qualifications and numbers of personnel that the procedure required, the necessary equipment, relationship to diagnostic facilities, and the minimum volume of procedures. Committee members felt so strongly about the need for teams performing the surgery to commit themselves to research that they devoted almost one-third of the 14-page report to criteria for assessing the results of surgery, including three pages devoted to diagrams directing autopsy. Already in 1975, Dr. Lepley was grieving over the disregard of the guidelines. He and other early developers interviewed by us in longitudinal personal interviews were to lament the role of money and other forces in driving a different diffusion pattern than that advised by the committee and to decry their successors' lack of interest in research.
I recalled those early days when I read the statement of the Committee on Fetus and Newborns of the American Academy of Pediatrics and pondered the fate of that document and the deliberations of the NIH workshop reported in this publication. Where do such guidelines fit into diffusion? Apparently statements are noted by recipients but not acted upon 18,19,20,21 In the following paragraphs I briefly consider the potential for guidelines to influence the categories of decision-makers that I have identified in this paper.
Medical Decision-makers: Adopters
Medical innovators, including early adopters, are likely to reject guidelines that are inconsistent with their own assessments of a technology. They embrace as goals the improvement of equipment, technique, knowledge, and results. They are aware of the changes that they and others have introduced and tend to dismiss contrary results, including controlled trials, as inapplicable because dated. Negative results from other teams are often attributed to incompetent performance of the technique. Innovators frequently disavow irresponsible diffusion of a new technology but contribute to it through their own demonstration and training, which provides the pool of professionals available to launch new initiatives.
Medical Decision-makers: Referrers
Education directed to individual physicians does not have a robust effect unless reinforced by local peers whose agreement may be quickened by pressures from patients, local media, manufacturers' representatives, and health care administrators. Potential referring physicians may be advised to evaluate ECMO facilities for desired characteristics but by the time they are considering a potential referral, they will probably have it associated in their minds with particular places and individuals known to them for this or other reasons. Since referring doctors do not decide appropriate application, additional information provided to them (if it has an effect) will likely stimulate referrals to ECMO teams for evaluation.
Fiscal and Managerial Decision-makers
In a situation of multiple payers, the opportunity to use payment to enforce guidelines seems limited. Fiscal incentives have proven effective enablers of expansion but poor vehicles of restraint. Competition among insurers has all but guaranteed coverage of high profile services atleast for certain groups. Competition among hospitals all but guarantees the pervasive development of these services at the expense of other services if need be. Payment decisions not in accord with local professional and public opinion present themselves not as the basis for a hospital's strategic plan but as obstacles to be surmounted in the interest of implementing it.
Strategic-Institutional Decision-makers
It is not clear at this time what potential ECMO has to be perceived by the public as a life-saving technique that should be offered by all excellent hospitals. Should it develop this image, it could be the sort of technology which is very hard to contain. Lack of sufficient volume for multiple programs is not necessarily a deterrent and may merely feed competitive rivalry to attract physicians with strong reputations or referral networks or to absorb losses as a cost of doing business. A hospital's strategic plan rests, finally, on the best guesses of administrators and boards of directors about future hospital care—the services which successful hospitals will offer and the ways they will be provided, including the standards which will be demanded by doctors and the public. Unless strategic decision-makers associate practice guidelines with operating or symbolic values as significant as the hospital's technology profile, they will be accorded minimal weight.
Conclusion
With dynamic technologies, those evolving as they diffuse, overall patterns of adoption and use of technology reflect a myriad of local decisions. These, in turn, reflect a variety of local considerations, both medical and non-medical. For many reasons guidelines which are externally developed may appear inapplicable and thus fail to achieve their desired results. Local social processes have shown themselves time and again to support their own versions of moral behavior, including sabotage of externally-imposed directives. In the United States, it is now unfashionable to ask physicians to shape the conduct of medical practice, except at the individual level. Nor have physicians come forth. The current system provides no consistent forum for local discussion of medical standards or priorities and no place for local physicians to participate meaningfully in the allocation of resources. Just as the adoption and use of innovation hinge on the action of local professionals, so may the restraint of innovation. Lest vitality and compassion be lost in the pursuit of rational medical behavior, local physicians need mechanisms for response which are as dynamic as the innovations.
Acknowledgments
This research is supported by the Agency for Health Care Policy and Research (Grant #RO1 HS 06065-01) which also supported the collection of baseline data (#1 RO1 HS 03238-01A1).
References
- Coleman JS, Katz, Menzel H: Medical Innovatiom: A Duffusion Study lndianapolis Bobbs-Merrill, 1966.
- Rogers EM: Diffusion of Innovations. New York, Free Press, 1983.
- Greer AL: Medical Technology Assessment, adoption, and utilization. J of Medical Systems 5:129-145, 1981.
- Greer AL: The state of the art vs. the state of the scinece: Diffusion of medical technology into practice. Int J. of Technology Assessment in Health Care 4:5-26, 1988
- Allen TJ: The role of person-to-person communications in the transter of technological knowledge. IN: Roberts EB, Levy RI, Finkelstein SN et al. Biomedical Innovation. Cambrioge, MA. MIT Press, 34-39, 1981.
- Paneth N, Wallenstein S: Extracorporeal membrane oxygenation and the play the winner rule. Pediatrics 76:622-623, 1985.
- Wung J-T, James LS, Kilohevsky JE: Management of infants with severe respiratory failutre and persistence of the fetal circulation, without hyperventilation. Pediatrics 76:488-494, 1985.
- Dworefz AR, Moys FR, Sabo B et al.: Survival of infants with persistent pulmonary hypertension without extracorporeal membrance oxygenation. Pediatrics 84:1-6, 1989.
- Donn SM: ECMO indications and complications. Hospital Practice 25:143-157, 1990. Personal communications, 1990.
- Greer AL: Adoption of medical technology: The hospital's three decision systems. Int. J of Technology Assessment in Health Care 1:669-680, 1985
- Fennell ML, Warnecke RB: The Diffusion of Medical Innovations: An Applied Network Analysis. New York, Pienum, 1-8, 1988
- Greer AL, Greer S: Medical Technology Decisions in Hospitals: Changes Since 1980. Final Report for Grant #R01 Hs 06054. The Agency for Health Care Policy and Research, Rockville, MD, 1991.
- Greer AL, Greer S: Some consequences of market forces in U.S. hospitals: Lessons for the new Look NHS?" Health Services Management, forthcoming 1990.
- Wagner JL, Krieger MJ, Lee RH et al: A Study of the Impact of Reimbursement Strategies on the Diffusion of Medical Technologies. Washington D.C., Urban Institute, 1982.
- Luke RD, Begun JW: The Management of Strategy, IN: Shortell SM, Kaluzny AD, Health Care Management: A Text in Organization Theory and Behavior, New York, John Wiley 463-487, 1988.
- Kosecoff J. Kanouse DC, Rogers WH et al.: Effects of the National Institutes of Health consensus development program on physician practice. JAMA 258:2708-13, 1987.
- Greer AL: The two coltures of bio-medicine: Can there be consensus? JAMA 258:2739-40, 1987.
- Lomas J, Anderson GM, Pierre KD et al.: Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med 321:1306-11, 1989.
- Lomas J. Words without action? The production, dissemination and impact of consensus recommendations. IN: Omenn GS (ed). Annual Review of Public Health 12:41-65, 1991.
Extracorporeal Life Support:
State-of-the-Art 1990
Robert H. Bartlett, M.D. and Charles Stolar, M.D.
University of Michigan Medical Center and
Columbia Presbyterian Medical Center
Introduction
The apparatus and techniques of extracorporeal circulation are routinely used for a few hours to permit surgery on the heart. With several modifications, extracorporeal circulation can be used for days or weeks to support the life of patients with severe cardiac or pulmonary failure. The procedure involves cannulation of major vessels without thoracotomy, carefully titrated partial anticoagulation with heparin, and continuous high-flow extracorporeal circulation through a membrane lung. Depending on the cannulation and application, this procedure has been called extracorporeal life support (ECLS), extracorporeal CO2 removal (ECCOR), extracorporeal heart assist, extracorporeal lung assist, and extracorporeal membrane oxygenation (ECMO). In this report the abbreviations ECLS and ECMO are used interchangably. ECLS is not a therapy, but a mechanical support system which allows time for the damaged heart or lungs to heal in a milieu of normal perfusion and gas exchange, while "resting" the damaged organs from the effects of mechanical ventilation and inotropic drugs. In the last decade ECLS has grown rapidly from a research protocol to clinical practice. It has been the most successful in neonatal respiratory failure and is now considered standard therapy for full-term infants with severe respiratory failure. It is the only method of mechanical cardiac support in children. It is a reasonable (albeit extraordinary) approach to the management of severe respiratory failure in children and adults.
Over 3,000 infant cases have been treated in the United States1 There are more than 60 neonatal centers offering ECLS as standard treatment.2 Currently the treatment is used for moribund infants with 83 percent survival overall and 95 percent survival in the most experienced centers: The incidence of pulmonary or neurologic handicap (approximately 20 percent)3 is lower than that of other neonatal intensive care unit (ICU) graduates.4 Two prospective randomized studies have demonstrated the effectiveness of ECLS in newborn infants,5,6 and two studies have documented an overall decrease in hospitalization and expense in neonate.7,8 Thus, although ECLS is the ultimate example of high-tech, labor and resource-intensive, expensive, invasive procedures, it routinely results in healthy children at less cost, resource utilization, and morbidity than the previous conventional treatment. (We wish this were true for liver and bone marrow transplantation or cancer chemotherapy.) A study group of active centers was organized in 1989—the Extracorporeal Life Support Organization (ELSO).
The results of ventilator and pharmacologic management in neonatal respiratory failure are excellent. Only a few neonates managed primarily at major centers fail to respond to treatment. There may be several deaths in any year if there are many cases of diaphragmatic hernia and neonatal sepsis. There may be none at all if only meconium aspiration and persistent fetal circulation are treated. Nonetheless, there are still approximately 3,000 deaths from respiratory failure in full-term infants each year in the United States. Almost all of these deaths are preventable. Why does extracorporeal support result in routine recovery of infants who are moribund with acute respiratory failure? Certainly there is nothing therapeutic about anticoagulation and extracorporeal circulation. Lung recovery must result from "resting" the lung from high pressure and high oxygen concentration. Simply by sustaining the life of the infant through a few days (which usually includes total lack of lung function) recovery of aeration, pulmonary blood flow and ultimate survival almost always results. This should suggest to us that there is something about the ventilator or pharmacologic management of this small group of full-term infants that contributes to pulmonary dysfunction. Obviously, a change in treatment aimed at preventing progression to severe respiratory failure would be better than treating established respiratory failure with ECMO.
Because most of the clinical application of ECMO is currently in newborn infants, this description and discussion will refer primarily to that group of patients. The basic principles of extracorporeal circulation, gas exchange, and systemic oxygen delivery apply to patients of all sizes and ages.
ECLS Technology
The circuit includes a servo-regulated roller pump, membrane lung, heat exchanger, tubing, and connectors (Figure 1). Right atrial blood is drained via a right internal jugular vein cannula to a small distensible bladder. A microswitch on the bladder automatically regulates the roller pump, controlling blood flow based on venous drainage and preventing air embolism. Blood passes through the pump and is perfused through a membrane lung. The size of the artificial lung is selected to provide total cardiopulmonary support, even though partial support will be adequate for most patients. Routine blood flow rates are 100–150 cc/kg/mm in neonates. As the blood is oxygenated, CO2 and water vapor are removed into the gas phase of the artificial lung. Blood then flows through a heat exchanger and back into the patient. In venoarterial circulation, the blood is perfused through the right carotid artery cannula into the aortic arch. In venovenous circulation blood is returned to the venous circulation.
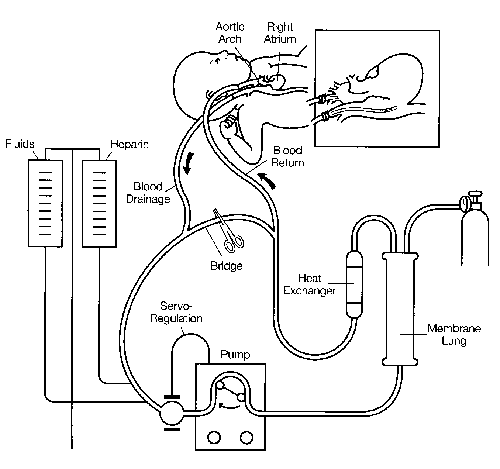
Figure 1. Diagram of a typical neonatal ECMO circuit. Venoarterial bypass is represented. Permission to reprint by Mosby Year Book, Inc. New York.39
Cannulation is performed at the bedside in the ICU with an operating room team present. The internal diameter of the venous catheter is the limiting factor which determines maximal flow. The diameter of the arterial catheter determines pressure in the circuit. Once on ECMO support, paralyzing agents, vasoactive drugs, and other infusions are generally discontinued. Ventilator settings are adjusted to minimal levels to allow "lung rest." Typical neonatal settings are: pressure limit 20 cm H20, positive end-expiratory pressure of 4cm H20, rate 10/mm, and FiO2 0.3. The patient is usually awake and alert.
During venoarterial bypass, blood flow is maintained at a level sufficient to keep the venous saturation at approximately 75 percent. Venous saturation is continuously monitored by a fiberoptic catheter in the venous line. A normal venous saturation insures that the combined oxygen delivery from the patient's cardiopulmonary system and the circuit is adequate for oxygen consumption requirements. A continuous non-invasive arterial oxygen saturation monitor is placed on the patient in an area of postductal blood flow distribution. With this monitoring available, arterial blood gases need only be drawn occasionally once the patient is stable. The arterial saturation is maintained at 95 percent and is manipulated by adjusting the extracorporeal blood flow rate. The PCO2 is maintained between 35 and 50 and is inversely proportional to the flow rate of gas ventilating the membrane lung.
During venovenous bypass, blood is recirculated to and from the right atrium, so that the mixed venous blood is typically 85–90 percent saturated. If the lung is not working at all the arterial blood will have the same saturation. As lung function improves there will be a step-up between right atrial and arterial blood. Although venovenous support results in lower arterial P02, systemic oxygen delivery is sustained by increased cardiac output, and the overall ability to provide lung rest and ultimate survival is the same as in venoarterial bypass. The use of venovenous bypass in the neonate avoids ligation of the carotid artery.
Heparin is infused continuously at 30–60 units/kg/hr. The level of anticoagulation is monitored hourly by the whole blood activated clotting time (ACT). The heparin dose is adjusted to maintain the ACT between 200 and 220 seconds (normal is approximately 100 seconds). There is a fall in platelet count at the onset of bypass; platelet consumption continues during ECMO. In infants, platelet transfusions are required to maintain a level greater than 75,000. The hematocrit is maintained between 45 to 50 percent and occasional red blood cell transfusion is required. In general, hemolysis is minimal and free serum hemoglobin 300 40 levels are usually <30 mg/dl during ECMO (normal <5 mg/dl). Antibiotics and parenteral nutrition are routine; diuretics are given if the patient is edematous.
The extracorporeal flow is gradually decreased when the native lung function increases. When the flow is approximately 20 cc/kg/mm a trial off the bypass at low ventilator settings is attempted.
If tolerated for a 1-3 hour period, the cannulae are removed. Patients often are weaned from the ventilator and extubated over the subsequent 24-48 hours. Management of a typical case is shown in Figure 2.
Figure 2. Ventilator settings and clinical events during a typical neonatal ECMO case. Permission to reprint by Mosby Year Book, Inc., Chicago, IL. In: Ravitch (ed.): Pediatric Surgery, 4th edition, 1986, pp. 74—77.
ECLS has become routine practice in the last several years because of the standardized system and approach, and because of the development of a new group of health care professionals—the ECLS clinical specialist. The need for ECLS specialists arose because the current systems require continuous attendance for monitoring and management, coagulation control, and management of emergencies. Specialists may have been trained in medicine, nursing, respiratory therapy, or perfusion. Extensive didactic, laboratory, and bedside experience is required, because even individuals from these various professions do not have the backgrounds necessary for ECLS management. The ECLS specialist team is essential for making the technique work, but it is also the most expensive component of extracorporeal life support.
The Development of Prolonged Extracorporeal Circulation
Between 1955 and 1970 hundreds of bioengineering and laboratory studies brought prolonged extracorporeal circulation from theory to clinical application. These studies are described in detail elsewhere.11,12,13,14
The first attempts at prolonged extracorporeal circulation in humans were by Callaghan,15 Dennis,16 and others. The first attempts at respiratory support in infants were reported by Rashkind,17 Dorson,18 and White.19 The first successful adult case was reported by Hill, O'Brien, and others in 1972.20 Reports of several other successful cases soon followed.21 22,23 In 1974 the Lung Division of the National Heart and Lung Institute proposed a multicenter, prospective, randomized study of ECMO in adult respiratory failure. This study began in 1975, which was a pivotal year for extracorporeal support.
In 1975 a meeting was held outside of Copenhagen which included most of the researchers on prolonged extracorporeal support; the proceedings were reported in a benchmark publication.24 The plans for the NIH adult ECMO study were reported and reviewed at that meeting. Four different membrane oxygenators were manufactured and used in 1975, the Kolobow Sci-Med, the LandJ -Edwards, the Pierce-GE, and the Bramson (the Food and Drug Administration did not become involved with devices until 1976). The first successful treatment of a newborn infant with ECMO was done in May 1975 and reported at the Copenhagen meeting.
Adult Respiratory Failure
The NIH-sponsored study of ECMO in adult patients was completed in 1979 and reported in 1980. 25 Other related studies of pathology findings and the epidemiology of respiratory failure26 in the study centers were reported. The study had a major influence on later prospective randomized controlled studies in neonates, so it will be described in some detail. This was the first attempt at a prospective, randomized study of a life-support technique in which the end point was death. There were many problems with the study; nine centers were involved, some of which had no prior experience with ECMO before their first study patient. The logistics of consent to the study tended to exclude the best-risk and worst-risk patients. A nationwide epidemic of influenza pneumonia occurred in 1976, and these patients dominated the trial. Bleeding complications were major, with average blood loss exceeding 2 L per day.28 Although the purpose of ECMO is lung rest, many of the patients remained on high ventilator settings.28 The study was planned for 300 patients, but it was terminated after 92 patients were entered because the survival in both control and ECMO group was less than 10 percent and it seemed unlikely that the results would be any different after 300 patients. The cause of death was related to technical complications in a significant number of patients, but extensive and apparently irreversible fibrosis was uniformly found at autopsy, indicating that the major problem was not the technology but the underlying parenchymal lung disease.26 As a result of this study, clinical research on ECMO in adult patients essentially stopped in 1979. Since that time only occasional cases have been reported in the United States, and the study of extracorporeal support in adults occurs primarily in Europe.
Evolution of the Concept of Extracorporeal CO2 Removal
Luciano Gattinoni worked with Kolobow at the National Institutes of Health, learning the techniques of extracorporeal support in sheep. He returned to Milan, Italy, with these hypotheses: (1) The purpose of ventilation is to excrete CO2; oxygenation can be achieved by inflation and airway oxygenation alone. Progressive lung injury in adult respiratory distress syndrome is caused in part by ventilator-induced high pressure injury of the most normal alveoli. When functional residual capacity is severely decreased, the remaining alveoli can be overinflated if high tidal volumes are used, leading quickly to alveolar injury and fibrosis. An extracorporeal support system should eliminate the need for high airway pressure and high FiO2, although this was not always done in the NIH-sponsored ECMO study. (2) If the emphasis is on CO2 removal to eliminate the need for high pressure ventilation, this could be accomplished with venovenous access, using relatively low flow and large membrane-oxygenator surface area. This system would allow for normal pulmonary blood flow, even if the lung is severely injured with large amounts of transpulmonary shunting. The venoarterial bypass used in the NIH ECMO study caused decreased pulmonary blood flow which might have contributed to microthrombosis or inhibition of lung healing.
Gattinoni and his colleagues used these principles in venovenous extracorporeal gas exchange in a variety of adult patients selected by the same criteria used for the NIH ECMO study. In 1986 they reported 21 survivors in 43 patients (49 percent).29 These results were corroborated by Lennartz and colleagues in Marburg, Germany,30 Falke in Dusseldorf,31 Bindslev32 in Stockholm, and Todd in Toronto.33 Similar results were reported by Morioka of Kumamoto, Japan.34 All of these investigators reported their results at a European communities conference held at Marburg, Germany in 1988.35
Neonatal Respiratory Failure
Bartlett, Gazzaniga, and their colleagues at the University of California, Irvine, treated the first successful neonatal ECMO patient36 (named by the nurses, Esperanza-Hope).37 This was soon followed by other successful neonatal cases.28 By mid-1980 they had treated 40 newborn cases with 22 survivors.39 The technique for newborn infants was fairly standardized, including venoarterial access via the right internal jugular vein and right carotid artery, heparin titration based on whole blood activated clotting times, "lung rest" at low ventilator settings, and recognition of persistent pulmonary hypertension as the primary underlying pathophysiology. In 1979 the first neonatal ECMO seminar was held at the University of California, Irvine, demonstrating the circuit, the technology, and the concept of the ECMO team, and specialists. This led to the development of ECMO research teams in Richmond, Pittsburgh, and Detroit. In 1980 the neonatal ECMO project moved from the University of California, Irvine, to the University of Michigan and experience gradually increased from a few cases each year to a few cases each month. Representatives of other centers attended the annual seminar, and some established ECMO teams—all with a standardized system and protocol. By the end of 1986 715 newborn cases had been treated in 18 centers40 with excellent survival results reported from each center.41,42,43,44 By the end of 1989 over 3,500 cases had been treated in 64 centers. The growth of neonatal ECMO centers and cases is documented in Figure 3. The sequence of events which controlled the diffusion of this technology are discussed in detail elsewhere in this symposium.
With the technique standardized and an experienced team trained, the Michigan group carried out a prospective randomized study in newborn infants between 1982 and 1984.45 They used a statistical technique called randomized play-the-winner, in which assignment to one treatment or the other is randomized, but influenced by all the previous patients in the study.45 Statistical significance is reached when there is a significantly larger group of patients in one arm of the study compared to the other. This resulted in the unusual groupings of one control patient (who died) and 11 ECMO patients (all of whom survived). This proved that the results with ECMO were better than conventional therapy, but the study was treated with skepticism. The most articulate of the critics, Ware and Epstein,49 undertook to design a prospective randomized study of ECMO in neonatal respiratory failure, but soon encountered the same problems of ethics and logistics. They solved this problem by using a similar adaptive statistical design. Four of 10 control conventional ventilation patients died and 1 of 38 ECMO patients died. This second prospective randomized study was reported in 1989.6
It should be noted that it is most unusual for a new technology to be tested by two separate prospective randomized controlled trials of life support systems in which the endpoint is death. These are, in fact, only the second and third such studies reported (the first was the adult NIH study referred to earlier). The ethical and logistical problems encountered when designing a prospective randomized study of a life-support technique in children are particularly difficult; this difficulty is compounded when it is known that at least 90 percent of the children given the experimental treatment will survive. During the adult ECMO study this problem and several other problems of study design were identified. All of these problems were addressed in the design of the neonatal studies.
Aside from a general increase in survival of patients considered to be moribund, the application of ECMO has had an impact on some specific neonatal conditions. Congenital diaphragmatic hernia symptomatic in the first day of life has carried a 50 percent mortality risk for the last 40 years. ECMO is used for those infants who are dying from pulmonary failure—the survival rate ranges from 60-90 percent in various centers. This has changed the natural history of diaphragmatic hernia from 50 percent mortality to 25 percent mortality, as documented in one recent study.47 Neonatal sepsis was thought to be a contraindication to ECMO in 1982. Septic patients are now treated routinely with 80-90 percent survival. Use of ECMO for premature infants less than 35 weeks gestational age was tabled in 1983 because of high incidence of intracranial bleeding in those patients. Now, with improved techniques of anticoagulation and management of intracranial venous and arterial pressure, research has resumed on respiratory failure in premature infants. The early results are very promising.
The follow-up on the first 72 neonatal survivors ranged from 3 months to 11 years.10 Ten children (14 percent) were lost to follow-up. Forty-five (63 percent) were normal or near-normal. Twelve (17 percent) had neurologic dysfunction and/or developmental delay. These results are similar to the findings of Towne,45 Krummel,49 and others who noted normal mental function in 70-80 percent of the patients in their studies. Additionally, all these results are comparable to the 77 percent normal mental ability noted in the 3-year follow-up of patients treated with conventional mechanical ventilation therapy. This suggests that neurologic damage is secondary to events that precede the onset of ECMO.
Eight of the first 72 neonatal ECMO survivors (10 percent) had residual lung disease when discharged from the hospital.10 The bronchopulmonary dysplasia (BPD) resolved in two patients. This compares with a 0-35 percent incidence of BPD in survivors of conventional therapy.
This increased survival in high risk neonates with relatively low morbidity is gratifying. However, ECMO also appears to have economic advantages as noted in a study by Pearson and Short.7 The average daily ECMO charge was approximately $4,000, almost $2,000 more than the daily charge for conventional therapy patients. However, the number of hospital days was substantially reduced in the ECMO patients (21 vs. 37.4 days). The total hospitalization charge of survivors in the ECMO group was 43 percent less than that of survivors among the conventional mechanical-ventilation therapy patients. A recent prospective, randomized study comparing cost effectiveness and morbidity of conventional ventilation to that with ECMO was recently conducted at the University of Michigan.8 ECMO patients had shorter hospitalization and less morbidity without increased hospital charges.
Present Status of ECLS
Patient Selection: Mortality Criteria, Indications, and Contraindications
ECMO is indicated when conventional management fails and the risk of morbidity or mortality is high. In respiratory failure the mortality risk is estimated by measuring the extent of pulmonary dysfunction and the level of ventilator support required to sustain gas exchange. In the neonate the underlying pathophysiology is usually pulmonary arterial vasospasm resulting in pulmonary hypertension and right-to-left shunting through the ductus arteriosus or foramen ovale (persistent fetal circulation syndrome, PFC). This is true regardless of the primary diagnosis. Because of this pathophysiologic syndrome, the degree of vasospasm and right-to-left shunting is reflected in postductal hypoxia despite high FiO2. In most neonatal centers PFC is treated by hyperventilation to induce alkalosis and relax the vasospasm. Compliance is usually decreased so that FiO2 and airway pressure are indirect measures of the degree of pulmonary dysfunction. These factors have been combined into measurements to estimate the severity of pulmonary dysfunction in the alveolar-arterial oxygen gradient (AaDO3) or the oxygenation index (OI). The normal AaDO2 is approximately 50 torr. When the AaDO2 is consistently higher than 600-620, despite optimal treatment, the mortality risk is 80-90 percent. The OI is calculated as mean airway pressure x FiO2 x 100/postductal PaO2. An oxygenation index consistently greater than 40 is generally associated with 80 percent or greater mortality risk. The usefulness of these measurements in any neonatal center is dependent upon the method and philosophy of ventilatory management. Each center must determine the mortality risk in that center using AaDO2, OI, or some other objective measurement.
Relative contraindications to ECMO in newborn infants are prematurity less than 35 weeks gestational age, preexisting intracranial bleeding or other major neurologic injury, or mechanical ventilation longer than 10 days. The incidence of severe bronchopulmonary dysplasia is high in children who have been ventilated with high pressures for a few days, and is prohibitively high in infants who have been ventilated more than 10 days. Intracranial bleeding may be exacerbated by anticoagulation, so ECMO is generally avoided in babies with ultrasound evidence of intracranial bleeding. However, the risk of extending a bleed can be minimized by decreasing the heparin dose and increasing the platelet count. Grade Ill or IV hemorrhage is a definite contraindication, but many experienced centers are now using ECMO for infants with Grade I and questionable Grade II intracerebral hemorrhage.
During the early development of neonatal ECMO, we realized that the incidence of intracranial bleeding was very high in premature infants (less than 35 weeks gestational age).51 Consequently, EGA less than 35 weeks became a contraindication in 1985, just before the rapid growth of cases and centers. Management of anticoagulation and blood flow has improved considerably, and the risk of bleeding is now decreased. The University of Michigan team recently used ECMO for two premature infants without complications. If this experience continues, prematurity will no longer be a relative contraindication.
Results
Between 1980 and 1989, 3,528 infants were registered in the Neonatal ECMO Registry of the Extracorporeal Life Support Organization. The patients were treated in 58 centers in the United States and 7 abroad (Table 1). The first 715 cases (1973-1986) from 18 centers were discussed in the first Registry report.40 The three years of 1987-1989 showed a 261 percent increase in ECMO centers and a 393 percent increase in treated infants. Although there was rapid increase in the aggregate and annual numbers of infants treated through 1988, the annual totals for 1988 and 1989 are the same (Figure 3). Similarly, the annual rate at which new ECMO centers have opened was greatest in 1987 and is less for 1988 and 1989 (Figure 4). The number of infants treated at any one ECMO center ranged from 2 to 100; the average annual number of ECMO cases was 12 per center per year. Almost all infants treated with ECMO were referred from a Level Ill neonatal intensive care unit specifically for respiratory management; 93 percent of the patients were outborn and only 7 percent inborn.
Table 1: Centers Contributing Cases to the Neonatal ECMO Registry (1990)
| Children's Hospital of Alabama, Birmingham, Alabama | Children's Memorial Hospital, Chicago, Illinois | Eastern Oklahoma Perinatal Center, Tulsa, Oklahoma |
| St. Vincent's Hospital, Birmingham, Alabama | Cook County Hospital, Chicago, Illinois | Emanuel Hospital and Health Center, Portland, Oregon |
| Phoenix Children's Hospital, Phoenix, Arizona | Lutheran General Hospital, Park Ridge, Illinois | Children's Hospital of Pittsburgh, Pittsburgh, Pennsylvania |
| St. Joseph's Hospital and Medical Center, Phoenix, Arizona | St. Francis Medical Center, Peoria, Illinois | Thomas Jefferson University Hospital, Philadelphia, Pennsylvania |
| Arkansas Children's Hospital, Little Rock, Arkansas | James Whitcomb Riley Hospital, Indianapolis, Indiana | Medical University of South Carolina, Charleston, South Carolina |
| Children's Hospital of Los Angeles, Los Angeles, California | Kosair Children's Hospital, Louisville, Kentucky | University of South Carolina, Columbia, South Carolina |
| Children's Hospital of Oakland, Oakland, California | Ochsner Foundation Hospital, New Orleans, Louisiana | John Sealy Hospital, Galveston, Texas |
| Children's Hospital of Orange County, Orange, California | Boston Children's Hospital, Boston, Massachusetts | Texas Tech University, Lubbock, Texas |
| Huntington Memorial Hospital, Pasadena, California | Massachusetts General Hospital, Boston, Massachusetts | Wilford Hall Medical Center, Lackland Air Force Base, Texas |
| San Diego Regional ECMO Program, San Diego, California | Johns Hopkins University, Baltimore, Maryland | Presbyterian Hospital of Dallas, Dallas, Texas |
| Sutter Memorial Hospital, Sacramento, California | Children's Hospital of Michigan, Detroit, Michigan | Primary Children's Medical Center, Salt Lake City, Utah |
| Stanford University Hospital, Stanford, California | University of Michigan Medical Center, Ann Arbor, Michigan | Medical College of Virginia, Richmond, Virginia |
| UCLA Medical Center, Los Angeles, California | Minnesota Regional ECMO Program, Minneapolis, Minnesota | Children's Hospital and Medical Center, Seattle, Washington |
| University of California, San Francisco, California | Cardinal Glennon Children's Hospital, St. Louis, Missouri | Children's Hospital of Wisconsin, Milwaukee, Wisconsin |
| Children's Hospital, Denver, Colorado | The Children's Mercy Hospital, Kansas City, Missouri | Assistance Publique Hospitauz de Paris, Paris, France |
| Children's National Medical Center, Washington, D.C. | St. Louis Children's Hospital, St. Louis, Missouri | Hospital Trousseau, Paris, France |
| Georgetown University Hospital, Washington, D.C. | University of Nebraska Medical Center, Omaha, Nebraska | Kumamoto University Medical School, Kumamoto, Japan |
| Miami Children's Hospital, Miami, Florida | The Babies Hospital, New York City, New York | Central Hospital, Kasugai, Aichi, Japan |
| University of Florida, Gainesville, Florida | Charlotte Memorial Hospital, Charlotte, North Carolina | Karolinska Institutet, Stockholm, Sweden |
| Arnold Palmer Hospital, Orlando, Florida | Children's Hospital Medical Center, Cincinnati, Ohio | Universitäts-Kinderklinik Mannheim, West Germany |
| Medical College of Georgia, Augusta, Georgia | Miami Valley Hospital, Dayton, Ohio | Royal Alexandra Children's Pavillion, Edmonton, Alberta, Canada |
| St. Luke's Hospital, Boise, Idaho | Rainbow Babies and Children's Hospital, Cleveland, Ohio | Children's Hospital, Columbus, Ohio |
ECMO Patient Description
The primary diagnoses for the group were meconium aspiration—39 percent; congenital diaphragmatic hernia—16 percent; respiratory distress syndrome—15 percent; persistent pulmonary hypertension of the newborn—13 percent; sepsis—12 percent; cardiac support—2 percent; and other—2 percent (Table 2). The distribution of diagnoses by year is shown in Figure 5. Although meconium aspiration syndrome predominates in all years, it has decreased from 47 percent of all ECMO cases in i985 to 37 percent in 1989 (p < .05). Over the same period of time there was an increase in the numbers being treated for sepsis (7 percent to 15 percent). The most common secondary diagnosis was PPHN (64 percent), followed by air leak syndrome (19 percent).
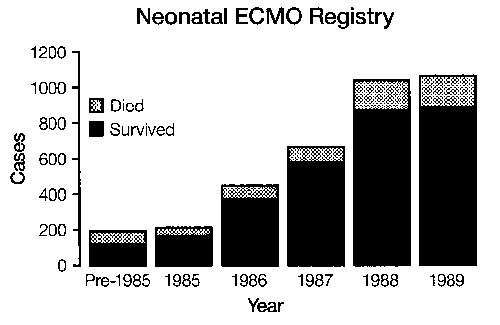
Figure 3. Neonatal ECMO cases and outcome during the development and clinical application stages.
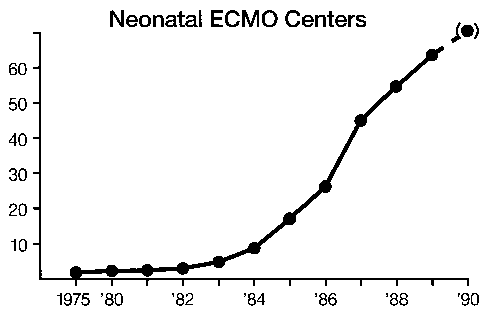
Figure 4. Neonatal ECMO centers/year.
Sixty percent were boys. Racial background, method of delivery, and payor status were incompletely reported. Mean 1-and 5-minute APGAR scores were 5.2 ± 3.8 and 7.2 ± 3.5 respectively. Mean birth weight was 3.2 " 0.6 kg and mean gestational age was 39 ± 2.4 weeks. The infants were 51.7 ± 48.6 hours old when ECMO was initiated and the mean duration of ECMO was 128.3± 111.6 hours.
ECMO Criteria
Criteria for instituting ECMO changed and evolved during the 10 years of this report. The goal of all selection criteria was to identify infants with a greater than 80 percent likelihood of mortality. The selection criteria were high (Table 3):
- alveolar-arterial oxygen gradient for 6-8 hours or more (22 percent)
- oxygenation index (18 percent)
- acute deterioration (14 percent)
- failure of maximal therapy (34 percent)
- barotrauma (1 percent)
- cardiac arrest (1 percent)
- no entry criteria indicated (10 percent)
The following occur just prior to ECMO (pre- or post-ductal not specified):
- Arterial blood gas measurements
pH=7.39 ± .20
PCO2=41.3 ± 23.8 torr
P02 =41.0 ± 32.3 torr
HCO3=22.6 ± 7.0 MEq/L
Sa02=70.6 ± 23.6% - Ventilator settings
rate/min=96.4 ± 71.6
FiO2=1.0 ± .06
peak inspiratory pressure=45.9 ± 10.9 cm H20
positive end-expiratory pressure=4.3 ± 2.8 cm H20
mean airway pressure=19.7 ± 4.8 cm H20
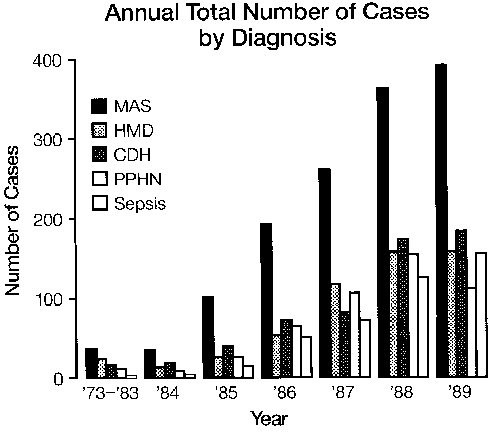
Figure 5. Diagnoses per year (Neonatal ECMO Registry Data).
The last pre-ECMO arterial blood gas analysis for each diagnosis is shown in Table 4. The following observations can be made:
- The P02 for hyaline membrane disease (45 ± 23 torr) is significantly higher than for other entry diagnoses (p < .05)
- The PCO2 (53 ± 24 torr) for congenital diaphragmatic hernia is significantly higher than for other entry diagnoses (p < .05)
- The pH for sepsis (7.29 ± .25) is significantly lower than for other entry diagnoses (p < .05).
ECMO Survival, Clinical Features
Overall aggregate survival was 83.1 percent (2,927 survived, 601 died) in a population of infants with an estimated likelihood of mortality of at least 80 percent.
Survivors and non-survivors differed in birth weight (3.3 ± 0.6 vs. 3.0 ± 0.7 kg), gestational age (39 ± 2 vs. 38 " 3 wks), and 5-minute APGAR scores (7 ± 2 vs. 6 ± 2). They did not differ in 1-minute APGAR scores and pre-ECMO ventilator settings. Survival for inborn infants treated with ECMO was less than for outborn (77 percent vs. 83 percent, p < .05). An infant weighing more than 2 kg and with a gestational age greater than 36 weeks had an 85 percent survival versus a 15 percent survival for infants less than 2 kg and 35 weeks gestation. The last pre-ECMO arterial blood gas analysis for survivors and deaths differed only in pH 7.41 ± .18 vs. 7.29 " .24 (p < .05). ECMO duration for survivors was 121 ± 60 hours compared to 147 ± 112 hours for deaths (p < .05). Additionally, patients undergoing major surgical intervention while being treated with ECMO, e.g., patent ductus arteriosus ligation or abdominal exploration, had only a 55 percent survival. These survivor characteristics are summarized in Table 5.
Table 2: Results Related to Diagnoses (Neonatal ECMO Registry 1980-1989, 3,528 cases)
| Percent Incidence | Percent Survival | |
|---|---|---|
| Meconium Aspiration Syndrome | 39 | 93 |
| Congenital Diaphragmatic Hernia | 16 | 62 |
| Respiratory Distress Syndrome | 15 | 84 |
| PPHN | 13 | 83 |
| Sepsis | 12 | 77 |
| Cardiac | 2 | 61 |
| Other | 2 | 77 |
Table 3: Selection Criteria (Neonatal ECMO Registry 1980-1989, 3,528 cases)
| Percent Incidence | Percent Survival | |
|---|---|---|
| AaDO2 | 22 | 89 |
| Oxygenation Index | 18 | 89 |
| Acute Deterioration | 14 | 76 |
| Failure of Maximal Therapy | 34 | 70 |
| Barotrauma | 1 | 90 |
| Cardiac Arrest | 1 | 52 |
| Other | 10 | 90 |
Table 4: Arterial Blood Gas, Pre-ECMO, by Diagnosis (Neonatal ECMO Registry 1980-1989, 3,528 cases)
| pO2 torr | pCO2 torr | pH | |
|---|---|---|---|
| MAS | 38 " 14 | 37 " 17 | 7.43 " .17 |
| HMD | 45 " 23 (p<0.05) | 38 " 14 | 7.40 " .14 |
| CDH | 31 " 14 | 53 " 24 (p<0.05) | 7.32 " .21 |
| Sepsis | 36 " 21 | 47 " 33 | 7.29 " .21 (p<0.05) |
| PPHN | 36 " 22 | 40 " 20 | 7.40 " .21 |
Table 5: Characteristics of Survivors vs Non-Survivors (Neonatal ECMO Registry 1980-1989, 3,528 cases)
| Survivors | Non-survivors | p | |
|---|---|---|---|
| Birth Weight (kg) | 3.3 | 3.0 | <.01 |
| Gestational Age (wks) | 39 | 38 | <.01 |
| Apgar (5 min.) | 7 | 6 | <.01 |
| PH | 7.41 | 7.29 | <.01 |
| pCO2 | .40 | 50 | <.01 |
| Outborn (%) | 83 | 77 | <.05 |
| ECMO Duration (hrs) | 121 | 147 | <.05 |
| Total number | 2,927 | 601 |
ECMO Survival by Selection Criteria
Survival outcome as a function of selection criteria used showed that both AaDO2 gradient and oxygenation index determination yielded an 89 percent survival. Patients treated because of acute deterioration had a 76 percent survival, suggesting the precipitous nature of their ECMO initiation. Although treatment of 34 percent of the patients for failure of maximal therapy (clinical judgement) might suggest a lack of rigorous selection criteria, this group had only 70 percent survival. This increased survival implies that the group was either more desperately ill or ECMO selection was more, not less, stringent than in the other entry criteria groups. Patients with other entry criteria had a 90 percent survival (Table 3).
ECMO Survival by Diagnosis
Annual survival rates for infants supported with ECMO increased from 56 percent in 1981 to 86 percent in 1987, and have remained statistically unchanged since (Figure 3). However, analysis of survival rate by diagnosis provides additional detail. Aggregate survival rates for the major entry diagnoses are listed in Table 2 (1980—1989): meconium aspiration syndrome—93 percent; persistent pulmonary hypertension of the newborn—83 percent; respiratory distress syndrome—84 percent; sepsis—77 percent; congenital diaphragmatic hernia—62 percent; and other—77 percent. Analysis of the effect of secondary diagnoses on survival outcome for the primary diagnoses demonstrated two significant associations. Survival for sepsis as a primary diagnosis was decreased from 77 percent to 63 percent (p < .05) when the secondary diagnosis was meconium aspiration syndrome, and survival for respiratory distress syndrome was decreased from 83 percent to 75 percent when the associated secondary diagnosis was sepsis (p < .05). Consideration of patient survival by entry diagnosis and year of entry is more revealing (Figure 6). Patients accrued through 1983 are consolidated as a group because of the small number treated compared to subsequent years. For all years of this report, meconium aspiration syndrome and persistent pulmonary hypertension of the newborn had an 83 percent—93 percent survival. Sepsis and hyaline membrane disease have improved significantly from 67 percent and 71 percent, respectively, to 79 percent and 88 percent (p < .05). Current survival rates for MAS, PPHN, HMD, and sepsis are statistically the same. These are to be distinguished from congenital diaphragmatic hernia (CDH). The highest survival rate for CDH was reported for 1987—70 percent. As of 1989, the annual survival rate for CDH had decreased to 56 percent. The 1989 decrease in annual survival for CDH was significant (p < .01) when compared to 1987, but also when compared to all other ECMO diagnoses (p < .05).
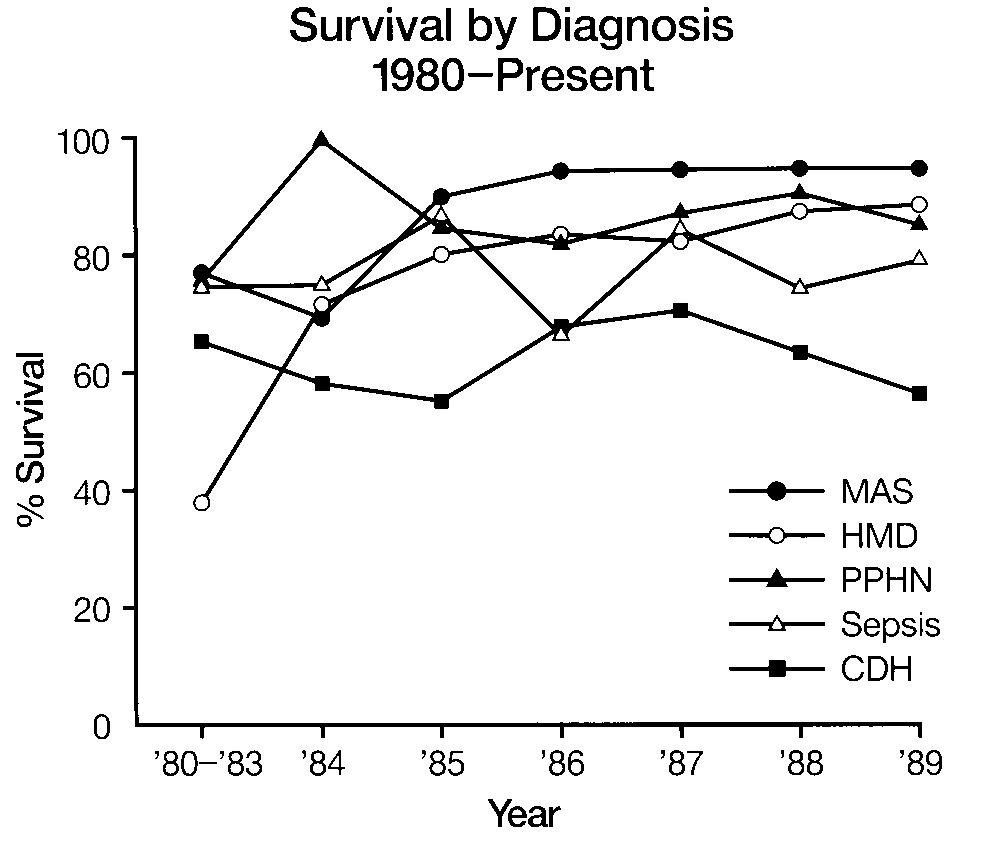
Figure 6. Survival related to primary diagnoses per year (Neonatal ECMO Registry Data).
Table 6: Technical Complications by Year (Neonatal ECMO Registry 1980-1989, 3,528 cases)
| Average number per patient | Percent with no complications | |
|---|---|---|
| 1980 | .12 | 73 |
| 1981 | .17 | 87 |
| 1982 | .37 | 69 |
| 1983 | .23 | 76 |
| 1984 | .47 | 65 |
| 1985 | .28 | 77 |
| 1986 | .23 | 80 |
| 1987 | .27 | 77 |
| 1988 | .27 | 76 |
| 1989 | .33 | 80 |
| Overall | .33 | 73 |
Table 7: Medical complications (Neonatal ECMO Registry 1980-1989, 3,528 cases)
| Percent Incidence | Percent Survival | ||
|---|---|---|---|
| Neurologic | Seizures | 15 | 68 |
| ICH | Ultrasound | 12 | 52 |
| CT | 3 | 83 | |
| Other | 3 | 66 | |
| Bleeding (non-ICH) | GI | 3 | 63 |
| Cannulation site | 10 | 67 | |
| Other surgical | 3.4 | 56 | |
| Lung | 0.2 | 63 | |
| Renal | Creatinine >1.5 | 9 | 62 |
| Dialysis/Hemofilter | 10 | 57 | |
| Cardiopulmonary | CPR required | 3 | 46 |
| Myocardial stun | 0.4 | 80 | |
| Arrhythmia | 3 | 66 | |
| Metabolic | Potassium <2.5 | 2 | 85 |
| Calcium <6 | 1 | 67 | |
| Glucose >240 | 0.8 | 48 | |
| pH <7.2 | 1 | 32 |
Technical Complications
Technical complications are listed in Table 6. In 3,369 ECMO cases 1,039 technical complications occurred, or 0.31 technical complications per infant treated. Technical complications were not evenly distributed throughout the Registry population. In fact, 76 percent of the entire group had no technical complications. The technical complication rate per patient decreased between 1964 and 1989 from 0.47 per patient to 0.33 per patient, while the portion of patients treated without technical complications increased from 65 percent to 73 percent. The most common technical complication was related to positioning of the cannulae (36 percent) which required repositioning for correction. Oxygenator failure represented an additional 24 percent of technical complications. Oxygenator failure was managed by either changing oxygenators while on bypass, continuing bypass with compromised oxygenator function, or termination of ECMO support. Although technical complications were not frequent, they did affect survival. Survival with one or more technical complications was associated with 80 percent survival compared to 84 percent survival without technical complications (p < .01). Surviving infants had .31 ± 0.5 complications per patients while non-survivors had .45 ± 0.6.
Medical Complications
Medical complications occurring while being treated with ECMO are listed in Table 7. There were 5,054 medical complications in 3,369 ECMO patients, or 1.50 complications per case. Medical complications per patient improved from 1964 (2.35 per patient) to 1989 (1.61 per patient). Neurologic complications predominated with 1,227/5,054 (24 percent). Survival of patients with neurologic complications was 735/1,227 (62 percent). Seizures and cerebral hemorrhage documented by either cranial ultrasound or computed axial tomography accounted for most of the neurologic complications (78 percent). Other hemorrhagic complications accounted for 1,065/5,054 (21 percent) of medical complications with survival of 67 percent. Cardiopulmonary complications accounted for 1,369/5,059 (17 percent) of medical complications. Survival averaged 63 percent, ranging from cardiopulmonary resuscitation required (45 percent) to systemic hypertension (81 percent). Renal complications accounted for 733/5,054 (14 percent) and were associated with a 60 percent survival. Half of the renal complications were managed by either continuous hemofiltration or hemodialysis. Metabolic complications accounted for only 423/5,054 (8 percent) of all medical complications. Although acidosis and hyperkalemia were associated with a significantly poorer survival than the aggregate population (32 percent and 50 percent vs. 83 percent), the relative infrequency of persistent metabolic derangements attests to the efficacy of ECMO in correcting abnormalities and maintaining physiologic homeostasis.
Overall, 37 percent of registered ECMO patients had no medical complications reported. Survival in patients with no medical complications was significantly higher than for those patients with one or more medical complications (95 percent vs. 76 percent, p < .01), as with technical complications. Surviving infants had 1.19 ± 1 medical complications while non-survivors had 3.11 ± 1. The improving complication rates for both medical and technical complications suggests improving safety of ECMO.
Diagnosis-Specific Complications
Significant associations between primary ECMO diagnoses and specific complications were limited to congenital diaphragmatic hernia and sepsis. Congenital diaphragmatic hernia (CDH)— compared to meconium aspiration syndrome, persistent pulmonary hypertension of the newborn, and sepsis—was significantly more commonly associated with hemorrhage (operative sites, gastrointestinal, other) and pulmonary vasodilator use (p < .01). Sepsis (compared to meconium aspiration syndrome, persistent pulmonary hypertension of the newborn, and congenital diaphragmatic hernia) was significantly more commonly associated with seizures, renal failure, culture positive infection, neutropenia, intracranial hemorrhage, and brain death.
The learning curve can be documented from the cases in the Registry. The overall survival is 83 percent. Survival for the first 10 cases from all centers is 78 percent, the first 20 cases 79 percent, and the first 30 cases 82 percent. The survival rate after 10 and 20 cases is significantly lower than the survival rate for 30 or more cases. This is related in part to experience of the ECMO team and in part to case selection (only the most critically-ill patients are treated early in the series).
Even with the regular addition of new ECMO centers, the overall survival rate has been unaffected and remains close to 90 percent for all entry diagnoses except congenital diaphragmatic hernia. Not only has the survival rate been sustained, the annual rate of technical complications has decreased over this time from .47 to .33 per patient, with an increased portion of patients reporting no technical complications. Similarly the annual rate of medical complications decreased from 2.35 to 1.60 per patient.
Diagnosis-specific outcome analysis suggests more about the particular diagnosis than ECMO, per se. Specifically, CDH had significantly more hemorrhagic complications and poorer survival than other diagnoses. Congenital diaphragmatic hernia, of all the ECMO entry diagnoses, is the only one which has less than two completely developed lungs and requires surgery. The hemorrhagic complications can be attributed to the major surgical operation to repair the diaphragmatic hernia, usually within 96 hours of ECMO initiation. The poorer survival can be related, in part, to the hemorrhagic complications, but also to pulmonary hypoplasia to a degree that is incompatible with life. Initially ECMO was used only for infants with evidence of adequate lung parechyma (a "honeymoon" period). In the last few years several centers are using ECMO for cases without a honeymoon. This results in treatment of more infants with irreversible pulmonary hypoplasia; subsequent deaths adversely affect survival statistics. Further, with increased availability of ECMO, more infants with CDH, particularly the most desperately ill, are being treated with ECMO.
Selection criteria remain problematic for a variety of reasons. They cannot be viewed as absolute because of variability between centers. What represents an 60 percent likelihood of mortality in one center may not be the case in another. Historical controls are misleading because changing respiratory therapy strategies make historical populations difficult to compare. Also, once an ECMO center becomes established, a more challenging group of patients will be attracted than might otherwise be seen. Further, as consideration of the last pre-ECMO arterial blood gas suggested, a single entry criteria cannot be generalized for all entry diagnoses. Eighty percent mortality criteria may be different for meconium aspiration syndrome, congenital diaphragmatic hernia, PPHN, and sepsis. Since ECMO is now considered standard practice for infants who fail conventional management (however that is defined), it is no longer possible to determine the mortality risk of conventional treatment. Since the survival rate with ECMO is over 90 percent in experienced centers, it is no longer necessary to determine specific mortality risk, only to determine that the risk of mortality or morbidity is high. Selection criteria are changing, as ECMO moves from "rescue" indications to "decreasing cost and morbidity" indications.
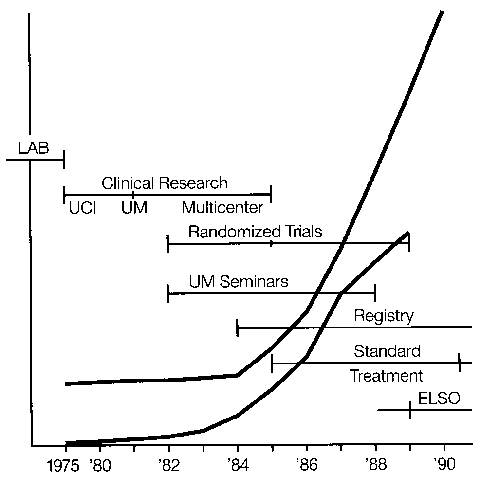
Figure 7. Events in the diffusion of ECMO technology. The curves indicate the number of centers and cases per year.
Subsequent patients registered in the Neonatal ECMO Registry of the Extracorporeal Life Support Organization will address these issues in considerable detail because many specifics of the pre-ECMO condition and therapeutic strategies are collected. Specific answers to these issues will be generated as large amounts of data are rapidly accumulated. Multicenter registry data yield information not available from a single institutional experience.
Neurologic and pulmonary status at discharge is difficult to determine from the Registry information, because many patients were transferred back to the hospital of origin after recovery from respiratory failure but before hospital discharge. From the information available, the incidence of abnormal neurologic status was 2 percent, abnormal hearing acuity 2 percent, and persistent pulmonary disease 10 percent. Follow-up studies from individual centers ranging from 1—10 years post ECMO indicate that approximately 70 percent of patients are normal at 1 year, and approximately 65 percent are normal at 3 years. The oldest survivor is currently 15 years old and is a normal healthy young lady. Recent follow-up and cost effectiveness studies are discussed elsewhere in this symposium.
The events which were important in the diffusion of neonatal ECMO from a single laboratory to general clinical application are outlined in Figure 7. This diffusion is discussed elsewhere in this symposium.
Future Research and Applications
Artificial Organs
In the future, variations of microporous membrane lungs with improved efficiency of gas transfer, lower perfusion pressures, workability of materials, and lower cost of manufacturer will replace the solid silicone rubber membrane. As heparin-bonded surfaces become available and the systemic heparin doses decrease, manufacturers will pay increasing attention to flow design, minimizing stagnant and eddy current zones while maintaining low resistance to blood flow. With these modifications only two sizes of membrane lung will be necessary: a neonatal/ pediatric size with a rated flow of approximately 1 liter per minute and a child/adult size with a rated flow of approximately 5 liters per minute.
The servoregulated roller pump is certainly the work horse of extracorporeal circulation but it is potentially dangerous. The centrifugal pump is equally dangerous because high negative pressure and hemolysis can easily occur. The ECLS pumps of the future will be passive filling, mechanically servoregulated, inexpensive, portable, compact, unable to aspirate or pump air, and durable. The Phone Poulenc/CoIlin Cardio pump currently used in Paris has most of these characteristics.52 Any passively-filling pump will require accurate on-line flow meters. Pump speed will be automatically adjusted to maintain adequate systemic oxygen delivery under a wide range of conditions, probably based on mixed venous saturation.
In the future almost all ECLS for respiratory support will be carried out in the venovenous mode, using a single catheter with two lumens53 or tidal flow systems.54 Venoarterial bypass will be used when cardiac support is required. Percutaneous access will become more common, using dilators to introduce peel-away sheaths through which the access catheters will be placed. The most important advance in the next decade will be in the development of nonthrombogenic prosthetic surfaces. These advances are important only when companies are able to routinely and reliably produce devices with specific coatings. Both Medtronic/ Carmeda55 and Bentley Laboratories56 have developed methods of surface bonding which permit ECLS without systemic anticoagulation in the laboratory. These systems will make it possible to conduct extracorporeal support with minimal or no systemic heparin. This will totally change indications, applications, and complications of extracorporeal support. Various aspects of the technique will have to be changed to allow continuous high flow, no bridges or luer locks, maintenance of flow around catheters in access vessels, etc. Although permanent surface bonding of heparin may minimize the platelet adherence, adhesion, and aggregation, heparin alone will not be the final answer to nonthrombogenic surface. Some combination of heparin, plasminogen activator, and prostacyclin analog, all bound to the prosthetic surface, will come closer to the normal endothelium, and may permit longer perfusion with lower flow rates.
Clinical Practice of ECLS
It will be interesting to see how the clinical supervision of ECLS is managed in the future. By the turn of the century it is likely that there will be a dozen patients on ECLS simultaneously in any major medical center, including premature infants, adult ventilator patients, and patients in cardiogenic and hemorrhagic shock. Common sense would dictate that one professional team should manage this system in conjunction with intensive care nursing. This team may be composed of a distinct new paramedical profession of ECLS specialists (as is currently the practice) or the responsibility might be ultimately taken on by subspecialties in perfusion, respiratory therapy, or nursing. The manpower requirements will be similar to current needs in major centers (three or four deep on-call schedules) but a typical work shift will involve priming circuits, emergency cannulations, elective decannulations, management of emergencies, and making rounds on the 10 or 20 ECLS patients for general supervision and preventive maintenance.
During the next decade the role of the ECLS specialist will change from continuous bedside supervision to simultaneous supervision of several ECLS patients, generally on an on-call basis. Minute-to-minute and hour-to-hour supervision of the ECLS system will be managed by the bedside nurse, much in the way that mechanical ventilators are managed now. Of course this will not be possible until the safety features outlined above are incorporated into the circuits, and until intensive care nurses are educated in the details of prolonged ECMO.
With improved circuit safety, single vein access, and minimal anticoagulation, indications for ECLS will change from moribund status to moderate respiratory failure. ECLS will become an adjunct to conventional ventilation and pharmacologic management, rather than something to try when standard ventilation and pharmacology is failing. ECLS will have a significant role for patients who are difficult to wean from venovenous (VV) mechanical ventilation. Low flow VV bypass (or intracaval gas exchange) will be used to facilitate extubation and allow ambulation, eating, and other activities that are often precluded by intubation and mechanical ventilation.
ECLS Spinoff
Many of the lessons learned in ECLS have already been applied to bypass for cardiac surgery (servoregulation, mixed venous saturation monitoring, membrane lungs, standardized descriptors of vascular access catheters). Heparin-bonded non-thrombogenic circuits will provide a major advance for cardiac surgery and other procedures in which extracorporeal circulation with circulatory arrest or control of local blood flow is desirable, e.g., dialysis, hemofiltration, and plasmapheresis. The technology of ECLS will be applied to normothermic organ perfusion.
ECLS has already led to better understanding of pulmonary pathophysiology. The identification of pulmonary hypertension in the newborn as the underlying pathophysiology in virtually all cases of full-term respiratory failure is one example. The study of ECLS has brought proper emphasis to the separation of oxygenation from CO2 removal, and the realization that high peak airway pressure during attempted hyperventilation for CO2 clearance is the major culprit in ventilator-induced lung injury. With this realization a return to low pressure mechanical ventilation will occur, with ECLS used as an adjunct when low pressure mechanical ventilation has not achieved CO2 clearance. The study of oxygen kinetics and the role of mixed-venous saturation during ECLS has already found its way to the routine management of ICU patients. In the ICU it is currently common practice to manipulate DO2 to optimize oxygen delivery/oxygen consumption (D02/V02) ratio. During ECLS it is easy to regulate V02 by regulating temperature, and this technique will find its way to routine ICU management.
The two prospective randomized trials of ECLS in newborn respiratory failure5,6 used adaptive statistical designs,45 bringing randomized clinical trial design to the forefront of discussion.46 This was particularly important because of the evaluation of ECLS as a life-support technique, so that ethical as well as statistical considerations guided the planning of these studies. Although initially criticized,46 both the conclusions and the methodology in these studies have stood the test of time and the use of adaptive designs will simplify prospective randomized studies in a variety of areas in the future.
Finally the general success of ECLS in newborn infants has brought the economics and ethics of high-tech intensive care to center stage. One prominent author questioned whether the cost of ECMO was justified to save a newborn life.57 (He estimated the cost at $25,000; it is actually about $15,000.) The question is certainly valid, although we commonly spend much more than that in the treatment of a single patient with AIDS, pancreatic cancer, newborn asphyxia, prematurity, and other conditions which have less favorable outcomes. A recent study demonstrated that length of hospitalization and hospital costs were actually decreased in ECLS patients compared to patients on conventional ventilation.8 Nonetheless, because ECLS is such a highly visible, complex technology, we will be— and should be—constantly asked, "Is it worth it?"
Clinical Applications
With the improvements in the system outlined above, ECLS will be applied earlier in respiratory and cardiac failure. It will be routinely used for premature infants, older children, and adults with respiratory failure from a variety of causes. ECLS will be used in conjunction with lung transplantation in two ways: to support the lung transplant patient through acute edema or a later rejection crisis, and as a bridge to lung transplant for children and adults with acute irreversible disease. ECLS will facilitate the study of living donor lobar transplant to small children.
ECLS will gain wider application as a temporary mechanical support of the circulation in children with cardiac failure. This application will be primarily for post-operative cardiac patients. Although ECLS can be used as a bridge to cardiac transplantation, the likelihood of getting a donor heart of appropriate size and blood type is small within the 2 or 3 weeks permitted by uncomplicated ECLS. Consequently, ECLS as a bridge to cardiac transplant will not be used until the donor supply is greatly increased (perhaps by ECLS as an organ perfusion system).
The application of ECLS in the management of trauma and resuscitation from hemorrhagic shock will come with the non-thrombogenic system. Exsanguinating hemorrhage—from a ruptured liver or a duodenal ulcer, for example— may be managed by simultaneous transfusion and volume replacement associated with quick cannulation for ECLS. Rapid cooling with perfusion will allow total circulatory arrest or continuous cold perfusion at very low flow rates to permit identification and repair of the bleeding vessels or organs, followed by re-warming on bypass.
This outline of prospects for the future of ECLS seems at best presumptive and at worst preposterous. However, in 1970 there had been no successful cases and it was widely held that prolonged extracorporeal support was impossible. By 1960 it had been demonstrated that successful ECLS was possible, but it was widely held that acute lung disease was irreversible in any patient sick enough to need it, and the technique, although possible, was impractical or unnecessary. In 1990 ECLS is standard treatment for some groups of patients. By now, experience has taught us to predict not the limitations but rather the possibilities.
Acknowledgments
Supported in part by grants from the National Institute of Child Health and Human Development and the William Randolph Hearst Foundation.
References
- ECMO Data Registry, Ann Arhor, Ml, 1990.
- Extracorporeal Life Support Member Centers, Ann Arbor, Ml. 1990.
- Glass F, Miller M. Short B: Morbidity for survivors of extracorporeal
membrane oxygenation: Neurodevelopmental outcome at 1 year of age.
Pediatrics 83:72-78, 1989. - Brett C, Dekl M, Leonard PD et al.: Developmental follow-up of
hyperventilated neonates: Preliminary observations. Pediatrics
68:588-591, 1981. - Bartlett RH, Roloff DW, Cornell RG et al.: Extracorporeal circulation in
neonatal respiratory failure: A prospective randomized study. Pediatrics
76:479-487, 1985. - O'Rourke PP, Krone R, Vacanti J at al.: Extracorporeal membrane
oxygenation and conventional medical therapy in neonates with persistent
pulmonary hypertension of the newborn: A prospective randomized study.
Pediatrics 84:957-963, 1989. - Short BL, Pearson GD: Neonatal extracorporeal membrane oxygenation:
A review. J Int Care Med 1:47-54,1986. - Schumacher R, Roloff DW, Chapman RA at al.: Neonatal ECMO: A
prospective randomized study of cost effectiveness. Submitted to ASAIO,
1992. - Bartlett RH, Gazzaniga AB: Extracorporeal Circulation for
Cardiopulmonary Failure. Current Problems in Surgery. Vol. 15, No. 5.
Year Book Medical Publishers, Chicago, 1978. - Bartlett RH, Gazzaniga AB, Toomasian JM at al.: Extracorporeal
membrane oxygenation (ECMO) in neonatal respiratory failure.: 100
cases. Ann of Surgery 204:236-245, 1986. - Kolobow T, Zapol W, Pierce JE at al.: Partial extracorporeal gas
exchange in alert newborn lambs with a membrane artificial lung
perfused via an AV shunt for periods up to 96 hours. Trans Am Soc Artif
Intern Organs 14:328, 1968. - Bartlett RH, Isherwood J, Moss RA et al.: A Toroidal flow membrane
oxygenator: Four day partial bypass in dogs. Surg Forum 20:152-153,
1969. - Landé AJ, Fillmore SJ, Subramanian V et al.: 24 hour venoarterial
perfusions of awake dogs with a simple membrane oxygenator. Trans
Am Soc Artif Intern Organs 15:181, 1969. - Hill JD, Bramson ML, Rapaport E et al.: Expermental and clinical
experiences with prolonged oxygenation and assisted circulation. Ann
Surg 170:448, 1969. - Callaghan JC, Maynes EA, Hug HR: Studies in lambas of the
development of an artificial placenta. Review of nine long-term survivors
of extracorporeal circulation maintained in a fluid medium. Can J Surg
8:208-213, 1965. - Dennis C: Certain methods for artificial support of the circulation during
intracardiac surgery. Surg Clin North Am 36:423, 1956. - Rashkind WJ, Freeman A, Klein D et al.: Evaluation of a disposable
plastic, low volume, pumpless oxygenator as a lung substitute. J of
Pediatr 66:94-102, 1965. - Dorson W, Jr. Meyer B, Baker E et al.: Response of distressed infants
to partial bypass lung assist. Trans ASAIO 16:345, 1970. - White JJ, Andrews HG, Risemberg H et al.: Prolonged respiratory
support in newborn infants with a membrane oxygenator. Surgery
70:288-296, 1971. - Hill JD, O'Brien TG, Murray JJ et al.: Extracorporeal oxygenation for
acute post-traumatic respiratory failure (shock-lung syndrome): Use of
the Bramson Membrane Lung. N Eng J Med 286:629-634, 1972. - Schulte HD: Membrane oxygenators in prolonged assisted
extracorporeal circulation. Dtsch Med Wochenschr 98:508. 1973. - Geelhoed GW, Adkins PC, Corso PJ, Joseph WL: CIinical affects of
membrane lung support for acute respiratory failure. Ann Thorac Surg
20:177-186, 1975. - Gille JP, Bagniewski AM: Ten years of use of extracorporeal membrane
oxygenation (ECMO) in the treatment of acute respiratory insufficiency
(ARI) ARII. Trans ASAIO 22:102, 1976. - Zapol WM, Qvist J: (eds). Artificial Lungs for Acute Respiratory Failure.
Academic Press, New YorK, 1976. - Zapol WM, Snicer MT. H JD at al.: Extracorporeal membrane
oxygenation in severe respiratory failure. JAMA 242:2193-2196, 1979. - Bartlett RH, Morra AH, Fairley HB et al.: A prospective study of acute
hypoxic respiratory failure. Chest 5:684-689, 1986. - Pratt PD, voilmer RT, Snalburn JD at al.: Pu monary morphology in a mult,nosp,ta. collaoorative ext racorporeal membrane oxygenation prolact. Am J Pathol 95:191-212, 1979.
- NHLDI-NIH: Extracorporeal Support for Respiratory Insufficiency. DREW Puolication, 1980.
- Gatfinon L, Pesenti A, Masoneroni D at al.: Low fraguency positive pressure ventilation witn extracorporeel D0 removal in severe acute respiratory failure. JAMA 256:881-885, 1986.
- Knoon M: Treatment of severe ARDS with extracorporeal DO.. remove,. IN: Neonatal and Aoult Respiratory Fe ~.ra. Gibe JR ledl. 6 sevier, Far a, 123-136, 1989.
- Falke K, Schulte HD: Ext racorporala DO~ aiim net on m nieor gfreguenter beatmung zur cehandlung des sonwern akuten wengenversagens. Dtsch Med Wocnenscnr 110:663-664, 1985.
- 8 ndslev L: Extracorporeal circ~ at on usng surface neparinizad eguipment. IN: Neonatal ann Adult Respiratory Farure. Glie JR ledl. E sevier, Paris, 97-1 00, 1989.
- Tond T: Personal commun cation, 1988.
- Morioka T, Terases, H: Present status of extracorporee ~ng ass at (EDLA as EDMO or ext racorporeal DO. ramovall in Japan. IN: Neonatal and Aoult Respiratory Fai,ure. G a JR (eel. Elsever, Paris, 147-157, 1989.
- Dartleft RH: Extracorporeal Lfe Support in Neonatal Reap ratory Failure. IN: Neonate. ano Adult Respiratory Fe ore. G .e JR ledI. Elsevier, Paris, 107-115, 1989.
- Dartlett RH, Gazzaniga AD, Jefferies R at a.: Extracorporeal membrane oxygenation IEDMOI card,opu monary s.~pport in infancy. Trans Am Soc Artif Intern Organs 22:80-88, 1976.
- Dartiaft RH: Esperanza IASAIO Presloent al Addressl Trans ASAIO 31:723-725,1985.
- Bartlett RH, Gazzaniga AD, Hoxtable HF at a.: Extracorporeal circu at on IEDMOI in neonatar respiratory fa,iura. J Thorec Dardioveac Surg 74:826-833, 1977.
- Dart aft RH, Anorews AF, Toomasian JM at a.: Extracorporae membrane oxygenat on IEDMOI for newoorn rasp ratory fa ~.ra: 45 cases. Surgery 92:425-433, 1982.
- Toomasian JM, Snenecor SM, Dome R at a.: Nat one exper ance w th extracorporee memorene oxygenat on IEDMOI for newborn respiratory failure: Data from 715 cases. Trans ASAIG 34:140-147, 1988.
- Krumme TM, Greenfied u. K repatricK Dv at el.: Dilnical use of an extracorporeal memorana oxygenator n neonatal go monary ta ~ra. J Pediatr Surg 17:525-531, 1982.
- Haroesty RL, Griffith DR. Deoski OF at a.: Extrecorporeal membrane oxygenat on: Successful treatment of persistent fete circuief on 10 ow ng repair of congen ta daphragmat c barn a. u Tnorac Daro oveac Surg 81:556-563,1981.
- Wenar TR, Penn ngton DG, Donnora R at al.: Extracorporee memorane oxygenation for newborn respiratory fa,,~.ra. Ann Thor Surg 42:529-535, 1986.
- Loe W, Graves F, Ochsner , at a.: Extracorporeal membrane oxygenation for nawoorn reap ratory failure. J Pen atr Surg 20:684-688, 1985.
- Dornell HG, .annenberger RD. Rartieft RH: Randomized play-tne-w nnar cinca tr ala. Dommun cat ons n Statistos: Tnaory and Metnoos 1:159-178,1986.
- Were JR. Epata n MF: Ext racorporee Dirculation in Respiratory Pa ~.re fcommenfariasl. Pan at rica 76:849-851, 1985.
- Hess K, Mann ng PD, Oldhem KR at al.: Reversal of mortality rate for DDH with EDMO. Ann Surg. 209:225-230, 1989.
- Towne RH, uotT IT, Hices DA, Healay T: Long-term follow-up of infants and chidran treated w th extracorporea membrane oxygenation IEDMOI: A pralim nary report. J Pedietr Surg. 20:41 0-4, 1985.
- Krummel TM. Greanfielo U, Kirkpatrick av at al.: The eary evaluation of survivors after axtracorporeal membrane oxygenaton for neonata pulmonary fa ure. J Ped,etr S.~rg 19:585-90, 1984.
- Anorews AF, Nixon DA, Rooff OW at a.: One-to-three year outcome of fo~rfeen neonate EDMO w..rvivora. Pao:atrica 78:692-698, 1986.
- Dilley RE, zwiscnanberger JD, Andrewa AF at al.: Intracrania namorrhage during ext racorporeal membrane oxygenation in neonates. Pediatrics 78:699-704, 1986.
- Durandy Y, Dheva.,er JY, Lecompta Y: Sing a cennule venovenous oypasa for respiratory membrane lung support. J Tnorac Dardioveac Surgery 99:404-409, 1990.
- Anderson HL. Otau T, Dnapman HA, Bartlett RH: venovanous ext racorporeal life support .n neonates ~a.ng a doub a lumen catnetar. Trans ASAIG 35:650-653, 1989.
- Kolobow T, Dorall M, Spatola H at a,.: S ogle catnetar venovano~s membrane ung bypass in the treatment of exparmenta ARDS. Trans ASAIO 34:35-38, 1988.
- Mottagny K, Oedasovan 8, Poppel K at el.: Haparin free long-term extracorporeal croulation ~sing 0 oactve surfaces. Trans ASAIO 35:635-635, 1989.
- Toomasian JM, Hau L-D, H rachi RD at al.: EvaL.et.on of Duraflo I. hepar n coating n pro onged ext racorporaa membrane oxygenation. Trans ASAIO 34:410-414, 1988.
- Philips JD: Treatment of PPHNS. IN: Fete, ann Neonatal cardiology. Long WA lad). Saunders, Philaoelphie, 691-701, 1990.
Neonatal Problems Treated with ECMO: Pathology, Prevention, Alternative Therapies
L. Stanley James, M.D.
Jen-Tien Wung, M.D.
Babies Hospital, Columbia Presbyterian Medical Center
Summary
The rapid proliferation of ECMO for the treatment of severe respiratory failure in newborn infants is causing a growing concern. Infants treated successfully with this technology have had a variety of clinical diagnoses, most of which are associated with persistence of the high pulmonary vascular resistance (PVR) present in fetal life together with pulmonary hypertension. The term persistent pulmonary hypertension of the newborn (PPHN) is now generally used to describe this condition when it occurs as a single entity and also to modify the description of the other disease entities with which it is associated, e.g., meconium aspiration or sepsis. It is characterized clinically by severe, labile hypoxemia with or without accompanying pulmonary parenchymal disease. The largest group of infants with these signs are born at or near term or are post-mature. They frequently have a history of an abnormal pregnancy, labor, or asphyxia at delivery. This group includes meconium aspiration syndrome with varying degrees of pulmonary parenchymal disease, PPHN with no meconium and little or no pulmonary parenchymal disease, transient tachypnea of the newborn, Type II respiratory distress syndrome (RDS), and severe respiratory distress in mature infants. Approximately two-thirds of the infants treated with ECMO are in this group.
Failure of the pulmonary vessels to relax their high fetal resistance after birth results in PPHN. The cause for this failure is not known but currently is thought to be due to either continued hypoxia after birth in an infant with normal pulmonary vasculature or to abnormal hypertrophy of the muscular pulmonary arteries in utero (the pulmonary hypertrophy caused by chronic hypoxia or pulmonary hypertension). There is controversy as to whether medial hypertrophy of the pulmonary arterioles is an essential component in the pathogenesis of PPHN. Dysfunction of the pulmonary vascular endothelium which has an obligatory role in the relaxation of vascular smooth muscle and which also plays a crucial role in the processing of vasoactive agents is likely to play a central role in this disease.
Hyperventilation with induced alkalosis is the most widely-practiced therapy for PPHN because of the belief that hypocarbia and alkalosis will lower PVR. However the improvement observed with hyperventilation in reducing PVR might well be due to the effect of improved oxygenation rather than that of hypocarbia or alkalosis as these several influences were not separately delineated in clinical studies. It has now been shown that a rise in PVR with hypoxia and acidosis cannot be prevented by hypocarbia and that the pulmonary vasoconstriction observed with hypercarbia is mediated through pH and not PaCO2. Furthermore, the evidence that alkalosis will lower hypoxia-induced PVR is contradictory. Hyperventilation has a number of additional adverse effects: the danger of barotrauma and air leak is increased and venous return may be impaired. Overventilation results in an increase in PVR and if prolonged can severely impair lung function.
The reported incidence of both meconium aspiration syndrome (MAS) and PPHN varies widely but can be lowered significantly with appropriate delivery room care. The currently used criteria for instituting treatment with ECMO based on 80 percent mortality risk need to be revised since there are now several reports of 80—100 percent survival of infants treated only with mechanical ventilation who meet these criteria.
There is a question as to whether excessive hyperventilation, so called maximal ventilatory support, contributes to the incidence of severe PPHN and the need for ECMO. A controlled trial comparing hyperventilation to a conventional (conservative) technique for the management of respiratory failure in newborn infants might provide an answer to this question.
With timely delivery room care and subsequent appropriate management of ventilation, severe PPHN should be a relatively rare event and most of these infants should be able to be managed by ventilation alone.
Introduction
The rapid proliferation of ECMO for the treatment of severe respiratory failure in newborn infants is causing a growing concern and raises a number of important questions. Does this reflect a true need, an increased availability of a new treatment for infants who would otherwise die, or are there more infants who require this care? Is the procedure being applied unnecessarily? Are financial incentives or the competitive needs for raising institutional prestige through introduction of the latest technology acting as a major force in propelling the increased use?
In this paper we will review our present understanding of the pathogenesis of PPHN, provide an approach to care that might serve as prevention and describe the current methods of ventilatory management being practiced. We will also suggest ways by which some of these questions might be answered.
Pathology
Conditions Treated with ECMO
Infants treated successfully with ECMO have had a variety of clinical diagnoses. From the ECMO registry1 of over 3,528 cases these clinical diagnoses fall into five main categories: meconium aspiration syndrome (MAS) 39 percent, persistent pulmonary hypertension of the newborn (PPHN) 14 percent, "hyaline membrane disease" (HMD) 15 percent, congenital diaphragmatic hernia (CDH) 17 percent, and sepsis 12 percent. The remaining 3 percent are classified as miscellaneous. All infants had been in severe respiratory or cardio-respiratory failure and could not be oxygenated using mechanical ventilation and pharmacological vasodilators. Irrespective of the various diagnoses, the underlying process in most these infants was a high pulmonary vascular resistance (PVR) and right-to-left shunting at the level of the foramen ovale and ductus arterlosus together with a low pulmonary blood flow.
Conditions Associated with a High Pulmonary Vascular Resistance in the Neonatal Period
Persistence of the high PVR with pulmonary hypertension after birth is a central feature in a wide variety of newborn illnesses in which there is cyanosis together with varying degrees of cardio-respiratory failure; some of these may be difficult to distinguish from congenital heart disease. The term persistent pulmonary hypertension of the newborn is now generally used to describe this condition when it occurs as a single entity and also to modify the description of other disease entities with which it is associated, such as meconium aspiration or sepsis. It is characterized clinically by severe labile hypoxemia with or without accompanying pulmonary parenchymal disease.
The largest group of infants with these signs are born at or near term or are post-mature and frequently have a history of an abnormal pregnancy, labor or asphyxia at delivery;2'3 meconium may or may not be present.4 This group includes MAS with varying degrees of pulmonary parenchymal disease,5,6 PPHN with no meconium and a little or no pulmonary parenchymal disease,7,8 transient tachypnea of the newborn (TTN),9,10 Type II RDS,11 and severe respiratory distress in mature infants.12 Among these different diagnoses, MAS contributes the greatest number of patients who are also the most severely ill and have the highest mortality. It is notable that there is often no relationship between the severity of symptoms, pulmonary arterial pressure, and extent of parenchymal disease by x-ray.5 In general the heart size in this group is normal, the lung vascular markings are either normal or slightly increased, and there is usually no evidence of heart failure.13 Approximately two-thirds of the infants treated with ECMO are in this group.1
A second group which also has a frequent history of abnormal pregnancy or birth is clearly in congestive heart failure with hypotension and poor arterial pulse amplitude in addition to respiratory distress.14 The heart is usually enlarged, vascular markings increased, and there may be tricuspid and/or mitral regurgitation.15,16,17 There is evidence that the heart failure is due to subendocardial schemia. Some overlap occurs between these two groups depending on the duration and intensity of the hypoxic stimulus, the tone of the pulmonary vascular bed and the duct, the state of myocardial integrity, and the extent of the collateral coronary circulation.13
In a third group, a high PVR develops as a complication of group B streptococcal sepsis and pneumonia.18 The high PVR is thought to be caused by the action of a polysaccharide toxin and increased levels of thromboxane A2 on the vascular smooth muscle.19
A fourth group consists of infants with developmental anomalies of the lung with restricted vasculature; it includes lung hypoplasia either as a discrete entity or in association with congenital diaphragmatic hernia.20 A miscellaneous group with an elevated PVR includes infants with polycythemia and hyperviscosity,26,22 hypoglycemia,23 or hypocalcemia. These conditions might best be regarded as complications of in utero hypoxia or asphyxia. Antenatal constriction of the ductus arteriosus causing pulmonary hypertension has been reported following administration of indomethacin or salicylates to the mother.24,25,26
Because of the variety of newborn illnesses associated with PPHN, the causes are likely to be multiple, both morphological and physiological. Consequently a particular syndrome associated with PPHN should not be considered as a single entity with a single cause.
Characteristics of the Pulmonary Arterioles and of the Fetal Circulation and Transitional Circulation at Birth
A high PVR is the normal characteristic of the fetal circulation during development; it is due to hypertrophy of the smooth muscle media of the pulmonary arterioles which have an increased tone in response to the low fetal PaO2. This serves to shunt most of the right heart output through the foramen ovale and ductus arteriosus to the systemic and placental vascular beds rather than to the lungs which receive only 7—10 percent of the cardiac output.27 While these characteristics are essential for the fetus, they pose serious hazards in the neonatal period; should they persist, they prevent the normal process of adaptation to extrauterine life, i.e., the establishment of an adequate pulmonary blood flow and the transition of the fetal pattern of circulation to that of the adult.
A rise in arterial oxygen tension after birth is of central importance in this transitional process, causing a decrease in PVR during the first 6 hours after birth and a gradual constriction of the ductus arteriosus. By 24 hours of age the healthy term infant has a functionally closed ductus arteriosus and a mean pulmonary arterial pressure substantially below that of the systemic arteries.28,29 In the weeks following birth there is a gradual regression of the muscular media of the pulmonary arterioles to the adult type. However, even in adult life these vessels retain their ability to constrict under hypoxic conditions and increase pulmonary arterial pressure by 15—20 percent.30 In contrast, a similar hypoxic stimulus in the newborn period can raise the pulmonary arterial pressure two- or threefold to reach suprasystemic levels, returning the circulation to the fetal pattern with the greater portion of the right heart ouput bypassing the lungs.31
PPHN results from a failure of postnatal relaxation of the high fetal pulmonary vascular resistance. This is currently thought to be due to abnormal in utero hypertrophy of the muscular pulmonary arteries (secondary to chronic hypoxia or pulmonary hypertension)4,6,8,32,33 or to continued postnatal hypoxia in infants with normal pulmonary vasculature.8,32,33 A prenatal pathogenesis is supported by the frequency of pregnancy complications in infants with PPHN2,3 as well as experiments in newborn rats which developed medial thickening of small pulmonary arteries in utero after chronic maternal hypoxia.34 Similar changes have been seen in the pulmonary vasculature of fetal lambs in which pulmonary hypertension was produced by premature closure of the ductus arteriosus or by constriction of the umbilical cord;35 indomethacin administration to the mother presumably causing prenatal ductus closure and pulmonary hypertension has also been implicated in PPHN.24,25 In infants who have died following PPHN or meconium aspiration, abnormal hypertrophy of the intra-acinar arteries and extension of muscle into small arteries has been described, again suggesting a prenatal origin.16 However this apparent hypertrophy of the pulmonary smooth muscle vasculature has been questioned on two accounts: because a vessel was not landmarked by its companion airway in order to distinguish hypertrophy or hyperplasia from mere contraction33 and in part because of non-uniformity of both the injecting technique used for preparation of the lungs and lung development.36
Continued pulmonary hypertension after birth has been shown to result in a secondary increase in arterial muscle in as little as 2 days.37 Furthermore there is experimental evidence in piglets that continued vasoconstriction can rapidly lead to deposition of connective tissue, which serves to fix the vessels in their constricted state and decreases their subsequent ability to vasodilate.38 These two factors, together with the demonstration that the pulmonary vascular bed appears to be more responsive to vasoconstrictive stimuli with increasing gestational age,39 could be responsible for the marked constriction seen in the MAS group (rather than in utero hypertrophy in these infants who are term or post-mature at birth).
Factors Influencing Pulmonary Vascular Resistance
Hypoxia and hydrogen ion
The pulmonary vasculature constricts in response to hypoxia.30,31 This effect is augmented by acidosis40 either by infusing acid or breathing higher concentrations of CO2. The response to both hypoxia and acidosis is much greater in the newborn period.31,41 Alveolar hypoxia appears to be a more potent stimulus of pulmonary vasoconstriction than arterial hypoxemia.42
Lung volume
Pulmonary vascular resistance is also influenced by lung volume. Fetal PVR decreases with lung expansion even when the level of oxygenation is maintained experimentally at the fetal level. At low lung volume PVR is high because the extra alveolar vessels become narrow; at high volumes the capillaries are stretched and their caliber reduced, increasing resistance. Thus PVR will be increased under conditions of hypo- or hyperventilation.43
Prostanoids
Metabolites of arachidonic acid include the most potent known constrictor and dilator substances in the lung circulation. These biologically active metabolites are produced in the lungs and are viewed as potential mediators of alterations in vascular tone and reactivity. Prostacylin (PGI2) is a pulmonary vasodilator. It is the major prostanoid released when perfused lungs are ventilated;44 when infused into hypoxic piglets with PPHN, PGI2 causes pulmonary vasodilation.45 However, PGI2 has not proven to be of value clinically. Thromboxane A2, a powerful pulmonary vasoconstrictor is elevated in PPHN of group B streptococcal sepsis.19
An association between pulmonary microthrombi or thrombocytopenia and PPHN has been noted46,47,48 suggesting that thromboxane, a proaggregatory substance might be released as a result of hypoxia or vascular endothelial injury and mediate both platelet aggregation and PPHN. Although thromboxane synthetase inhibitors appear to prevent both the rise in thromboxane and pulmonary vasoconstriction in the newborn animal model,49 clinical application in the human has not been fruitful. Thus, while there is some evidence that disturbances in vasoconstricting and vasodilating metabolites of arachidonic acid could be playing a role in the development of PPHN, vasodilators or cyclo-oxygenase inhibitors so far have not proven to be helpful in the treatment of PPHN.
Endothelium-derived relaxing factor (EDRF)
The observation that endothelial cells have an obligatory role in the relaxation of vascular smooth muscle by acetylcholine was first reported nearly 10 years ago.50 The importance of this finding has recently led to a growing interest with regard to the role of the endothelium in modulating the tone of the underlying smooth muscle in response to pharmacologic agents, physiologic stimuli, and disease.51 The endothelial cells release a dilator substance, EDRF. This has been shown to be nitric oxide, produced by the metabolism of L-arginine.
The pulmonary endothelial cells play a crucial role in the processing of vasoactive agents (e.g., biogenic amines, prostaglandins, and peptide hormones). This role is mediated by specific enzymes, enzyme inhibitors, receptors, or transport molecules situated on their luminal surface.52 Disease or injury of the vascular wall results in impairment of endothelium-dependent relaxation. When endothelial cells regenerate after mechanical trauma there is a reduced endothelium-dependent responsiveness to aggregating platelets and serotonin.53 Under certain conditions, endothelial cells can also initiate contraction of underlying smooth muscle. This activity becomes prominent under acute pathologic conditions (e.g., hypoxia or reperfusion injury). The opposing actions of relaxing and contracting factors released from the endothelium can therefore have far-reaching effects on vascular tone.51
Recently it has been demonstrated that hypoxia causes a significant change in the characteristics of the endothelial cells, compromising cellular barrier and coagulant functions.54 Cells become larger and exhibit intercellular gaps with marked changes in permeability, facilitating edema formation. In addition, expression of a direct Factor X activator develops under hypoxic conditions; the activator is membrane-associated.
These recent advances in our understanding of the functions of the pulmonary vascular endothelium carry important implications for our future understanding of PPHN. Injury to this endothelium or altered function as a result of hypoxia may explain why the vasodilating prostinoids have to date not been found useful therapeutically in this condition.
Prevention
Infants with MAS and PPHN form the largest group treated with ECMO. Approximately 70 percent of infants who develop severe PPHN have a history of complications during pregnancy, labor, or delivery. Some of these infants are in cardiorespiratory difficulty from the moment of birth. Because our knowledge of the factors involved in the circulatory transition at birth and the incomplete maintenance of a low and stable PVR, preventive measures must of necessity focus on those factors that are known to have an effect, notably good lung expansion and adequate oxygenation. The value of the presence of personnel experienced in newborn resuscitation in the delivery room cannot be overestimated. A transitional care nursery within the delivery suite where all aspects of emergency intensive care procedures can be carried out is ideal.
The most difficult cases are asphyxiated infants who have aspirated meconium. We first initiated immediate removal of the meconium by tracheal suction in 1960.35 Since that time we have had a low mortality and a relatively low incidence of cardiorespiratory difficulties in this group. In the past 7 years, in an inborn population of 31,000 deliveries including a large proportion of high-risk pregnancies, we have had no deaths and only 14 mature infants with severe respiratory failure and PPHN, an incidence of less than 1 in 2,000. All 14 survived with conservative management (Table 1) and none required ECMO. This low incidence of complications is likely to be related to both the immediate care at birth and the subsequent management of respiratory difficulties.
Table 1: 61 Infants with Severe PPHN (Babies Hospital 1983—1990)
14 Inborn: All Survive–No ECMO
(31,000 Deliveries)
47 Outborn: 44 Survived
35 No ECMO–1 Died (Parents Refused ECMO)
12 ECMO–2 Died (Brain Death)
Immediate Care at Birth
If meconium is present at delivery, it is removed manually with a gauze pad followed by suctioning. After delivery further meconium, which may be present in the trachea, is removed by direct endotracheal suction prior to ventilation. This procedure should be carried out in all infants in whom there is meconium. If the infant does not breathe spontaneously after tracheal suctioning, the lungs are expanded mechanically with high concentrations of oxygen. For the next 30 minutes close attention is paid to the infant's oxygenation; chest physiotherapy and pharyngeal suctioning, if necessary, are used to ensure continued clearing and removal of meconium. An arterial PaO2 and pH is obtained as soon as possible after birth, since skin color in these infants is a very unreliable indication of oxygenation due to peripheral vasoconstriction. If the infant's PaO2 is less than 50–60mm Hg and significant acidosis is present, e.g., a pH less than 7.25, blood gas monitoring should be continued until the level of 50–60mm Hg is achieved. Suboptimal oxygenation during this critical period may cause the pulmonary vasculature to remain constricted, contributing to the development of PPHN.
If breathing is established promptly, the endotracheal tube is removed. Thereafter the infant should be carefully observed for the next 2–3 hours. Should labored breathing or intercostal retractions be present, nasal continuous positive end-airway pressure (CPAP) is begun. All procedures are carried out in a warmed crib under radiant heating because of the importance of maintaining body temperature. Care should be taken not to overheat the infant as this will increase the metabolic requirements and may also cause peripheral vasodilation, systemic hypotension, and the maintenance of the fetal pattern of the circulation.
On the average, moderate or thick meconium is present at birth in 10 percent of our deliveries. This incidence is similar to that generally reported from other institutions. Most of these infants, even those who aspirate thick meconium, are relatively asymptomatic after appropriate immediate suctioning at delivery, followed by chest physiotherapy and further suctioning if necessary in the transitional nursery. They are then admitted to the regular nursery. Since 1974 these procedures for meconium-stained infants have been recommended by several groups.56-61 The procedure was endorsed by the American Academy of Pediatrics in 1977.62 One comparative study demonstrated a significant reduction both in mortality and morbidity57 and the largest survey of 176,790 liveborn infants found the incidence of MAS and deaths from the disorder to have declined significantly with the use of these procedures.61 The reported incidence of MAS among infants who have meconium present at birth ranges from 1:2361 to 1:76.56 At Babies Hospital this incidence is 1:70 and only 23 percent of these infants have required treatment with mechanical ventilation, an incidence of approximately 1 per 2,000 consecutive births.
The most important risk factors for MAS include postmaturity, maternal toxemia, prolonged labor, thick or "pea soup" meconium, moderate to severe fetal heart rate deceleration, and low scalp pH.63 They have been associated with the greater likelihood for the development of severe MAS.64 Nevertheless there are a number of reports of MAS with thin meconium staining. Although there has been some dis-agreement as to the need for routine suctioning,66-69 in the balance it would appear to be prudent to suction all infants at risk. While these procedures may not entirely avoid the development of cardiorespiratory difficulties or MAS with PPHN, the weight of evidence indicates that they will reduce both mortality and morbidity. With appropriate treatment in the delivery room and in the early neonatal period, the incidence of MAS should be in the range of 1 per 70 infants with meconium present at birth and the need for mechanical ventilation in the order of 1 per 2,000 consecutive births.
Alternative Therapies
The management of mature infants with PPHN and respiratory failure is extremely difficult and presents one of the major challenges to the neonatologist. These seriously ill infants are extremely labile and withstand any stress or handling poorly. The reason for their extreme lability is not understood but might be multifactonal. It could be related to the abnormal vasoconstriction secondary to hypertrophy of the smooth muscle media of the pulmonary arterioles.8,32,33 Alternatively, it could be related to the failure of the normal relaxation of pulmonary vascular tone due to an absence or decrease in the amount of the endothelial-relaxing factor,50,51 secondary to damage to the lung parenchyma from hypoxia or barotrauma. Intrapulmonary shunting and abnormal distribution of ventilation leading to hypoxia probably also play a role. Recently it has been demonstrated in an experimental model of pulmonary hypertension in sheep, that granulocyte accumulation in the lungs (which also occurs in group B streptococcal sepsis with PPHN) plays a role in the hyperreactivity.70'71 Granulocyte depletion attenuates both the sustained pulmonary hypertension and the increased pulmonary vasoreactivity.
Hyperventilation
Hyperventilation with induced alkalosis is the most widely practiced therapy for PPHN. The technique was introduced in 1978 in the belief that hypocarbia and alkalosis could lower PVR."72,73 In an effort to achieve a "critical" level of hypocarbia and alkalosis, hyperventilation is instituted, frequently with high peak pressures and a high rate of ventilation. The recommended protocol is to use a respiratory rate of 100 to 150 breaths per minute and whatever peak inflating pressures are necessary to decrease arterial PCO2; pressures as high as 70 cms H20 may be needed.73
Hyperventilation has a number of adverse effects. Not only will the danger of barotrauma and air leak be increased, but venous return may be impaired, particularly in those infants whose myocardial function is already compromised from hypoxia. If the procedure leads to over-ventilation and high lung volume, the capillaries are stretched and their caliber reduced, increasing PVR.43 Furthermore, overventilation can severely impair lung function.74,75 Ventilation of healthy paralyzed and anesthetized sheep with peak inspiratory pressures of 50 cms H20 has resulted in death from respiratory failure between 2 and 35 hours.
The initial improvement which may be observed in the infant with PPHN when hyperventilation is initiated could be the result of a rise in PaO2 as lung volume is increased and not to hypocarbia.76 In the original clinical observations the effect of lowering PaCO2 was not distinguished from the effect of raising the PaO2.72,73 If the infant is being overventilated, the initial improvement is likely to be followed by deterioration due to an increase in PVR from overinflation accompanied by damage to the lung parenchyma and/or pneumothorax, impairment of venous return, and eventually respiratory failure. Barotrauma to the lung parenchyma is likely to include damage to the pulmonary endothelium with all the adverse consequences associated with a loss of EDRF.51
Survival following hyperventilation reported by the group originally proposing this technique was 50 percent.77 More recent reports of survival from centers where less aggressive hyperventilation appears to have been practiced have been 89 percent to 100 percent.3,78 Higher mortality is associated with significantly higher peak inspiratory pressures, longer total times on O2 with FiO2 greater than 80 percent, and the development of pneumothorax.79
Hypocarbia following hyperventilation can have an adverse effect on the cerebral vasculature and circulation80,81 and, if excessive, can lead to ischemia.82 Although the neurological outcome following hyperventilation has been favorable in some reports,3,83 it has been associated with sensorineural deafness84,85 and an increase in neurological impairment if prolonged.86 There is considerable experimental evidence that the pulmonary vasoconstriction observed with hypercarbia is mediated through pH and not PaCO2. Earlier experiments in both man and dog have shown that higher levels of hydrogen ion produced by either raising PaCO2 or infusing acid cause an increase in PVR.40 Acidosis serves to augment the increase in PVR caused by hypoxia. Later experiments in newborn calves41 confirmed the additive effect of hypoxia and acidosis in raising PVR, the effect being much greater than had been observed in adult dog and man. The effect of hypoxia was overriding, an increase in PVR occurring in the presence of a normal, elevated, or low PaCO2. The evidence that alkalosis will lower hypoxia-induced PVR is contradictory. In man, alkalinization with either TRIS buffer or sodium bicarbonate neither lowers PVR nor prevents a rise in PVR with hypoxia.40 On the other hand, alkalosis has been shown to attenuate the hypoxia-induced pulmonary vasoconstriction in newborn lambs87 but there was no evidence that hypocarbia could prevent a rise in PVR with hypoxia and acidosis.
Thus these experiments, as well as observations in man, provide no support for the thesis that hypocarbia per se will lower PVR.
Conservative Ventilation
At Babies Hospital we have adopted a relatively conservative approach for the treatment of respiratory distress following aspiration of meconium or conditions which are associated with PPHN. Treatment is focused on maintaining adequate oxygenation and minimizing barotrauma while the disease process resolves. This has enabled us to manage the majority of infants with severe PPHN without using ECMO.
The principles of our management are to provide graded assistance to ventilation depending upon the degree of respiratory distress or failure; treatment is initiated early, as soon as signs of respiratory difficulty are apparent. In order to minimize barotrauma, respirator settings are kept at a low level compatible with adequate oxygenation and no attempt is made to induce hypocarbia or to keep the PaCO2 in the normal range by increasing the level of ventilation. Infants are not paralyzed. These principles apply to both the immature infant suffering from RDS and more mature infants suffering from MAS or PPHN.
Early Nasal Prong CPAP
For infants with respiratory distress, we initiate early treatment with nasal prong CPAP. This approach may avoid the subsequent need for more invasive mechanical ventilation. Indications for CPAP are tachypnea, inspiratory retractions, and nasal flaring with or without an audible grunt. CPAP is maintained at 5 cm of water pressure. Higher pressures usually result in air escaping through the mouth. The concentration of inspired oxygen is adjusted to keep the arterial oxygen tension between 50 and 70 millimeters of mercury. As soon as the apparatus is properly applied, the baby will breathe more easily; respiratory rate and retractions will decrease. If 5cm H20 CPAP is not sufficient to achieve a PaO2 of 50—70 mm Hg while breathing 80 to 100 percent oxygen, the patient probably requires mechanical ventilation.
Mechanical Ventilation
The major complications of mechanical ventilation are the result of barotrauma and adverse effects on the circulation. In order to minimize these complications our approach has emphasized having modest but adequate ventilator settings. The intermittent mandatory ventilation (IMV) mode allows spontaneous breathing to continue during the expiratory phase of the respirator cycle and permits the gradation of ventilatory support depending upon the degree of respiratory failure. Hence, the ventilator cycling frequency can be set at a lower rate for the same level of minute ventilation. This reduces the danger of barotrauma and cardiovascular compromise and at the same time facilitates weaning.
Indications for mechanical ventilation are marked retractions, while on CPAP, a PaO2, of less than 50 mm Hg, with an FiO2 of 80 to 100 percent, a PaCO2 greater than 65 mm Hg, or an intractable metabolic acidosis with a base deficit greater than 10 meq per liter despite bicarbonate therapy. Four methods of mechanical ventilation88 have been practiced in our neonatal intensive care unit over the past 8 years. The particular method employed will depend on the infant's response.
- Conventional method: This type of ventilation is based on physiological principles of traditional pulmonary mechanics. Most of the neonates with severe respiratory failure are ventilated using this method. Flow rate should be enough to generate the desired peak inspiratory pressure within the inspiratory time. We arbitrarily set a flow rate of 7 liters per minute. FiO2 is adjusted to maintain an arterial oxygen tension greater than 50 mm Hg. IMV rate depends upon the infant's ability to breathe spontaneously. The initial setting is usually between 20 to 30 breaths per minute and is then adjusted to maintain the PaCO2, between 50 to 60 mm Hg; the rate should be sufficient to avoid the infant's need to make excessive respiratory efforts which will lead to exhaustion. The infant soon adapts to this and breathes spontaneously during the expiratory phase of the respirator cycle. A higher level of carbon dioxide than normal is accepted because it allows a lower level of minute ventilation while maintaining adequate oxygenation. Most of our infants are ventilated with inspiratory time of 0.6 seconds. Peak inspiratory pressure (PIP) depends upon the compliance of the lung. It is adjusted so that adequate but not excessive chest excursion is visible, indicating that the tidal volume is adequate. Usually it is in the range of 20 to 30 cm H20. Lower pressures are used for the less-severely ill infant whose compliance is higher. If the inspiratory pressure is too low, tidal volume will be inadequate and the infant will develop atelectasis and intrapulmonary shunts leading to hypoxemia. If, on the other hand, the inspiratory pressure is too high, the lung will be overinflated. This leads to three important consequences: notably, increased lung injury from barotrauma; an increase in pulmonary vascular resistance; and circulatory compromise with decreased pulmonary blood flow, cardiac output, systemic blood pressure, and oxygenation. Positive end-expiratory pressure (PEEP) is usually set at 5 cm H20.
- High frequency positive pressure ventilation (HFPPV): This method is tried for those infants who fail to respond to the conventional method if (a) PaO2 is less than 50 mm Hg on FiO2 100 percent, (b) PIP is higher than 35cm H20 or (c) PaCO2, is above 70 mm Hg with an IMV rate of 40. For these infants the IMV rate is increased to 100 and the inspiratory time reduced to 0.3 seconds. The peak inspiratory pressure is lowered by 5 cm H2O from the conventional setting while the PEEP is set at zero. The flow rate is increased to 10–20 liter per minute (LPM). If the patient's condition improves, as indicated by the blood gas status or a more stable PaO2, these settings are maintained.
- Prolonged inspiratory time: If the infant cannot maintain an adequate PaO2, using the conventional or HFPPV settings, we return to the conventional method and increase the inspiratory time (Ti) from 0.6 to 0.8–1.0 seconds. Infants who require this type of ventilation have very stiff lungs due to such conditions as congenital pneumonia or severe RDS.
- IMV rates of 40–100 per minute: For a vigorously breathing infant who has not improved using either the conventional or HFPPV method, an MV rate of 40–100 is tried. The IMV rate is set at the infant's spontaneous respiration rate in an attempt to synchronize with the infant's breathing.
Weaning
An attempt to wean the infant from mechanical ventilation is begun as soon as the infant is stable. The FiO2, is lowered in decrements of 2 to 10 percent to maintain a PaO2, between 50 and 70 mm Hg. The IMV rate is lowered in decrements of 2 to 5 per minute, maintaining the PaCO2, between 50 to 60 mm Hg and allowing spontaneous but not excessively labored breathing. PIP is lowered as the patient's pulmonary compliance improves and chest excursions become excessive.
If the patient was best ventilated at a rate of 100, weaning is accomplished by lowering the FiO2, and PIP as indicated, leaving the IMV rate at 100 until reaching a PIP of 20 cm H2O. The management is then changed back to the conventional method with the IMV lowered to 40 per minute. Thereafter the infant is weaned as in a conventional technique. For the patient being ventilated with prolonged Ti, the Ti is gradually decreased to 0.6 seconds. Weaning is then continued as in the conventional method. For the infant on an MV between 40 and 100 the IMV rate is lowered and Ti changed accordingly as the infant's condition improves.
Muscle Relaxants
We discourage the use of muscle relaxants to paralyze the infant for a number of reasons. There is a better match between ventilation and perfusion with spontaneous breathing.89 For the paralyzed patient lying supine, the upper portion of the lung is ventilated more while the lower dependent portion is better perfused. Adequate ventilation can be achieved at a lower rate in a nonparalyzed patient breathing spontaneously, thus reducing the risk of barotrauma to the lung. Long-term paralysis can result in atrophy of the muscles of respiration, making weaning from mechanical ventilation more difficult. An additional important point is that the clinical status of the infant can be assessed by his/her spontaneous activity. For those infants referred from other centers and in whom the PaO2, may have been low for an unknown period of time, spontaneous activity and tone provide valuable guides in the assessment of hypoxic insults to the brain.
Recording of Transcutaneous P02
Continuous recording of the transcutaneous oxygen tension or saturation is essential during mechanical ventilation. This enables trends to be followed more readily and the infant's response to a change in the ventilator setting to be observed immediately, allowing the physician to determine promptly whether the new ventilator settings are in the right direction. This is particularly important for the very unstable infa nt and may help in diagnosing the cause of deteriorating blood gases. Furthermore, considerable fluctuations in the PaO2, are not unusual in patients with PPHN; continuous recording of the PaO2, will reveal whether or not the fluctuation is transient, thus avoiding unnecessary changes in the ventilator settings.
Persistently High PaCO2
Occasionally a patient's PaCO2, remains elevated despite a high respirator rate and adequate chest excursions. This is probably due to a high physiological dead space or V/Q disturbances. Attempting to lower the PaCO2 by increasing the ventilator settings is not beneficial and will only serve to inflict lung damage. Should a pneumothorax occur as a result of the high ventilator settings the infant's condition will promptly worsen. An elevated PaCO2, can be tolerated if hypoxia is not a problem. Infants with hypercarbia that is refractory to increases in IMV may often be weaned by decreasing the IMV without further elevation in PaCO2,. As the patient's pulmonary status improves with time, the PaCO2, will gradually fall.
For patients with PPHN, no attempt is made to induce alkalosis by hyperventilation or by continuously infusing alkali. Alkalosis will shift the oxygen hemoglobin dissociation curve to the left which may impair tissue oxygenation. This situation is especially dangerous for the patient with a low PaO2, Furthermore, as noted above, evidence for a beneficial effect of alkalosis is conflicting.40,87
Tolazoline
If the infant remains hypoxic despite proper mechanical ventilation with good chest excursions, tolazoline is given intravenously in a bolus injection of 1 mg/kg, while the PaO2 is recorded continuously with a transcutaneous electrode. If a beneficial effect is demonstrated after the initial bolus, a continuous infusion of tolazoline is given at 1 mg/kg per hour. Tolazoline has been reported to improve oxygenation76,90,91,92 in 40 to 87 percent of cases through its vasodilating effect on the pulmonary vasculature. Our experience is in agreement with these earlier reports, a rise in PaO2, being observed in approximately 90 percent of cases following the administration of the drug. However, in infants who are being hyperventilated the response is variable.72 Failure to respond to tolazoline could be due to overinflation which leads to pulmonary vasoconstriction, this effect overriding the vasodilating action of the drug. It could also be due to impairment of venous return, leading to a systemic arterial pressure which is lower than the pulmonary arterial pressure.
Mean Airway Pressure
The widely held belief that PaO2, is directly proportional to mean airway pressure (MAP)93 may create problems in the management of ventilation. This concept is only partly true since MAP is altered by any change in flow rate, IMV, Ti, PIP or PEEP. The value of PaO2, will depend not only on the respirator settings but also on the infant's cardiac and pulmonary status. Progressively raising MAP in response to a falling PaO2, will adversely affect oxygenation in the presence of circulatory failure or overinflation of the lungs.
Incidence of PPHN
Respiratory distress in mature or post-mature infants, including those associated with aspiration syndromes, TTN, and Type II RDS, are of relatively common occurrence and account for nearly 30 percent of admissions to our NICU. This proportion of admissions is similar to that obtained by the National Perinatal Information Center from a random selection of 25 NICUs nationwide and is probably representative of the national experience. Thus a relatively large group of infants are potentially at risk of developing PPHN and becoming candidates for ECMO. Both TTN and Type II RDS should have a very low mortality. Furthermore there are at least five reports of a high survival rate from MAS or PPHN in infants meeting the current ECMO criteria but without the use of ECMO,3,78,94,95,96 indicating that these current criteria are not predictive of an 80 percent mortality and should be revised. Indiscriminate intervention with excessive hyperventilation in this group of mature infants with various forms of respiratory distress could carry the potential danger of converting an illness with a low mortality into a serious one necessitating ECMO. It is of interest that in one report the mortality from PPHN fell dramatically with the avoidance of hyperventilation and the introduction of less aggressive ventilatory techniques.95
The reported incidence of PPHN in consecutive deliveries varies considerably3,61,90 from 1 in 425 to 1 in 2,000 in our own institution. Furthermore, the incidence of PPHN in intensive care unit (ICU) admissions has been reported to be as high as 1 in 43 in a children's hospital referral center which did not have an ECMO unit at the time.97 At Babies Hospital it is 1 in 350 in in-born infants and 1 in 80 when infants referred for ECMO are included. These large differences among various institutions are not likely to reflect different patient populations but rather differences in methods of management.
As noted earlier, we have diagnosed PPHN in only 14 of 31,000 births in our inborn service over the past 7 years. All 14 survived with conservative management (see Table 1). During the same 7-year period 47 outborn infants with severe PPHN have been referred to our unit for ECMO (excluding those with congenital diaphragmatic hernia). These infants were in very poor condition upon admission with evidence of overventilated lungs following mechanical hyperventilation. Nevertheless 34 were successfully treated by ventilation alone, gradually recovering as the respirator settings were lowered. Thirteen of the 47 had deteriorated to such a degree on admission that they met our criteria for ECMO. Twelve were placed on ECMO. Of the 47 outborn infants, 3 died; 2 were declared brain dead while on ECMO and in one the parents refused permission to place the infant on ECMO. Thus over the past 7 years our overall survival among 61 infants with PPHN who were in severe respiratory failure is 95 percent, the majority being treated by ventilation alone.
Criteria for ECMO
Criteria for the use of ECMO was initially based upon retrospective analysis of patients treated prior to 1981 and predicted a high mortality rate without ECMO.98 Criteria currently being used are based on the infant's ability to oxygenate, as reflected in the alveolar-arterial oxygen difference (AaDO2)99 or other parameters.100 Again a high mortality without ECMO has been predicted by these criteria. These criteria apply to infants being treated with hyperventilation. As noted earlier, the survival of 80 percent to 100 percent of infants being treated with mechanical ventilation alone indicated that those criteria should be revised.
Our criteria for ECMO require that the infant have a PaO2, less than 40 mm Hg for at least 4 hours while receiving 100 percent oxygen, or that the PaC2, is in the low 40's and is unstable requiring greater than 45 cm H2O PIP in 100 percent oxygen, or that there is sepsis with an intractable metabolic acidosis.88 Additional prerequisites for bypass are that the infant should have none of the generally accepted contraindications; has, where possible, been given an optimal trial of mechanical ventilation; and has failed to respond to tolazoline. The major difference between our criteria and those generally used99,100 is our use of conservative ventilatory methods rather than hyperventilation.
Two controlled trials have been conducted to determine the benefits of ECMO over "conventional" medical therapy.98,101 Unfortunately, "conventional" ventilation was hyperventilation with all the adverse effects enumerated above. These trials, therefore, demonstrate the superiority of ECMO, but only over maximal hyperventilation. Infants with PPHN who have been treated with our methods of mechanical ventilation have not required a prolonged hospitalization. Neurological follow-up has revealed a good outcome102 and none of the infants have been found to have sensorineural deafness.
The wide variation in the incidence of MAS and PPHN noted in this review strongly suggests that there are considerable differences in the approach to care and the management of these infants. It raises the possibility that severe PPHN might be in part an iatrogenic disease as a result of inadequate resuscitation and care at birth and subsequent overactive intervention with hyperventilation—so called maximal ventilatory therapy. This possibility is supported by the low incidence of MAS and PPHN in institutions where immediate care is managed by experienced personnel102 and the recovery of infants who meet ECMO criteria when the ventilator settings are lowered. The question might be resolved by epidemiological surveys together with a controlled trial comparing hyperventilation with optimal ventilator therapy.
The dangers of high peak airway pressures continued over many hours is well documented.75,104,105 The description of the course following this treatment in animal experiments is not unlike the clinical description of infants with severe PPHN. "There was a deceptively unremarkable early course; subsequently there was a progressive deterioration in pulmonary function, and alteration in lung structure leading to acute respiratory failure."75 Kolobow has equated this end point to the transitional phase PPHN and has questioned whether this is the result of treatment or the natural course of the disease.106
The case histories of some of the infants referred to our center for ECMO therapy read like a horror story with peak pressure of >60 cms H2O for hours, excessive administration of fluids to combat hypotension leading to increase in weight of up to 2 kilograms, plus bicarbonate, THAM, tolazoline, pressor agents, Pavulon, and steroids. Remarkably, most of these infants respond to lowering of ventilator settings and ceasing the excessive use of pharmacology. In others the pulmonary status is so compromised that they are unable to respond and are placed on ECMO. Equally remarkable, these infants respond within a few days to a period of lung rest. However, it would be a serious misapplication of technology if ECMO were to be used because of our inability to apply preventive care at birth and appropriate support of ventilation for those in respiratory difficulty.
Over a 7-year period, at Babies Hospital, 14 cases of severe PPHN occurred in 31,000 consecutive deliveries. Applying this statistic to the 3 million births per year in the United States would indicate approximately 1,354 infants are born each year with severe PPHN. With appropriate delivery room care and subsequent management of ventilation in the NICU, serious PPHN should be a relatively rare events58-60,94,95,107,108 and most of these infants should be able to be managed by ventilation alone.
Acknowledgments
Supported by the National Institute of Child Health and Human Development and Ronald McDonald Children's Charities.
References
- National EDMO Rag stry Report. 610 Ann..ai EDMO Sympos ..m, Breceenridge. Doloreno February-March 1990.
- Drummond WH, Peckham GJ, Fox WW: Tne c n ca profile of the newborn wiTn pulmonary hypartans on. D .n Padiatr 16:335-341, 1977.
- John F, Roberts v, Burnard ED: Pars stent p.. monary hypertension of toe newborn treated w Tb hyparvant let on: D n cal features and outcome. A..st Paediatr J 24:357-361, 1988.
- Murphy JD, Han novitch M, Goldstein JD, Red .M: The str..ct..rei basis of persistent pulmonary hypertens on of the nawoorn ntant. J Reoatr 88:962-967, 1981.
- Fox WW, Oawtz MH, Dinwidnie H at al.: P.. monary nypertension in tne perinata asp rat on synoroma. Pan etr ca 59:205-211, 1977.
- Murpny JD, vawter GE, Red .M: Puimonery veac.. er disease in fetal meconium asp rat on. J Red atrlO4:758-762, 1984.
- Gersony WM, Duo Gv, Sino air JD: RED syndrome (persistence of tne fatal c roulationl. Dim, at on 4O:fSuppl il~, 87A. 1969.
- Gersony W: Neonatal p.. monary hypartenson: Ratnopoys -o ogy, ciessit cation ann at ology. D n ca n Per netology 11:517-524, 1984.
- Avery ME, Getewood 00. Drumley 0: Transient tachypnee of tne newborn. Am J Os Dhilo 111:380-385,1966.
- Bucciereili RE, Egen EA, Gassnar at al.: Pars stance of fete cardiopulmonary dm0 at on: One manifestation of Trans ant tecnypnee of the newnorn. Pediatrics 58:192-197, 1976.
- Sunda H, Garroft ,, Diensenship WJ at al.: Stunies in intents witn type I rasp ratory distress syndrome. J Pen atr 78:754-764, 1971.
- Robertson NRC, Haiiioie-Smitn KA, Davis JA: Severe respiratory distress mim cking cyanot 0 heart disease in term nab as. Lencet2:1108-1110, 1967.
- Rowe HO: Abnormal p.. monary vesoconstriction in tna newborn. Pediatrics 59:318-320, 1977.
- Durnard ED, James LS: Fe ore of the neert after undue asphyxia at birtn. Pediatrics 28:545-565, 1961.
- Rowe OD, Hoffman T: Transient myocerdia schemia of the newborn infant: A form of severe cardioresp,ratory distress n full-term nfants. J Peo atr 81:243-250,1972.
- Lay n DL, Haymenn MA, Kittarmen JA at al.: Persistent p.. monary hypartens on of the newborn. J Pedietr 89:626-630. 1976.
- D..cc,ara HL, Na son HM, Egan EA at el.: Transient tric..spid ns..ft denny of the newborn: A form of myocardie dysfunction in strassan nawnorns. Paoiatr ca 59:330-337, 1977.
- Roes J, Green RS, He arpvisT DO at al.: St..dias on group B Date-hemo yTic streptococcus. ii. Effects on pulmonary nemodynem os ano vesco ar permeability n unaneatnetizad sheep. Rediatr Has 15:899-904, 1981.
- Kohl PG. Dotton RB, Schweer F, Saybarth 8W: Enoogannus formation of prostano da n neonates witn pars stent hypartans on. Arcn D a Dn 064:949-952,1989.
- Gegga HL, Murpny JO, .engiebian D at el.: Dongen ta diepnregmatic barn a: Arterial-structure changes end persistent pulmonary hypertans on after s..rgicei rape r. J Pen etr 107:457-459, 1985.
- Gatti PA. M..ster Au, Dole RH, Pa.. MH: Neonatal poycythamia witn transient cyanosis end cardioresp,ratory ennormalit as. J Pan etr 69:1063-1072, 1966.
- Siassi H, Go dbarg SJ. Emmano.. des GD at el.: Persistent po monary veac.. ar obstruction in newborn infants. J Radiatr 78:610-615, 1971.
- Amatayeko 0, Dumm ng OH, Haworth JD: Assoc at on of hypoglycemia with cardiac enargement and neert ta ..ra in newborn infants. Arch Die Dhild 45:717-720, 1970.
- Manonester D. Margolis H, She non H: Possible essoc at on between maternal inoomethacin therapy ano pr mary po monery nypertans on of the newborn. Am J ObstaT ann Gynac 126:467-469, 1976.
- Deaba . Sulyos F, Pot DT: He ationship of meterna treatment witn ndomaThec n to persistence of fetal c mcuietion syndrome. J Red atr 92:484, 1978.
- Runoiph A: Effects of asp rin ano acetaminophan n pregnancy ana the newborn. Arcn intern Med 141:358-363, 1981.
- Born GVR, Dewes OS, Mott ..D, W odnombe JO: Dhengas in The nearT and lungs at b rth. Dold Spr ng Harbor Symp O..ant Bin 199:102-108, 1954.
- Rowe HO, James LS: The normal pulmonary arteral pressure ourng Tna first year of fe. J PediaTr 51:1-4, 1957.
- Moss AJ, Emmanouiiioes GD, Butte ER: Diosore of the ouctus erTar osus in tne newborn infant. Pad atr d5 32:25-30, 1963.
- Fienmen AR, MeDement J. H mine stan A, Do,.mnano A: Effects of acuTe anoxia to tne c reuletion end respiration in patients witn chronic pulmonary disease st,.d ad d..rng The staaoy state.' J Dun invastig 31:770-781,1952.
- James ES, Howe HD: The paTtern of response of po monary and system c arterial prassjras n newnorn and older nfants to snort periods of nypoxia. J Pedietr 51:5-11,1957.
- Ruooiph AM: Hign pulmonary vasci am resistance after birtn. D n Pan atm 19:585-590, 1980.
- Geggel H, Red EM: The str..dt..rai basis of PPHN. Dun PerineToi 11:205-211,1984.
- Goiooarg SJ, Levy, HA. Siassi H, Rattan J: Effects of materna nypox a and hypamoxie upon the neonatal go monamy veaduieTure. Rao.atr cs 48:528-533, 1971.
- Lay n DL, Hymen Al, Haymann MA, Runoiph AM: Fete hypertension ann the development of indreased pulmonary vascular smooth muac a: A POSS o a mechanism for parsstant po monary hypertension of the newnorn nfant. J Pediatr 92:2365-269, 1978.
- Penman EJ, Moore OW, Hutdhins GM: The po monary vescuieture in maconium asp rat on. Human PeTho ogy 20:701 -706, 1989.
- Myr dk uD, Reid EM: The affects of continued hypoxe on reT pulmonary artery circ.. at on: An u Tine structura st..dy. Lab invest 38:188-200, 1978.
- HaworTh 5: Pulmonary vasdu am remodeling in neonatal pulmonary hypertension, chest (supp 193:133-138,1988.
- Hodo pn AM, Heymann MA: Pulmonary dirdulation in tata amos. Pan etr Has 6:341A, 1972.
- Hargofsky EH, Lenin DE, Fisnman AR: The affect of cnanges n hydrogen on concentration on the pulmonary c rduiat on. J Dun invest 41:1492-1502, 1962.
- Rudo ph AM, Yuan 5: Response of tna pulmonary vesd..etore to nypoxie ann hydrogen-on cnangas. J D n invest 45:399-411, 1966.
- Galantow rz ME, Price M, Stolar DJH: D fferential affects of eveolar ano arteria oxygen tension on poimonary vasomotom tone in EDMO pertused isolated piget lungs. 6th Annual EDMO Symposium. J Pan atm S..rg 26:312-5, 1991.
- West JD: Respiratory Physiology - The Essent ais, 3rd Ed. Hatimore, MD, Williams and Wilk ns, 38, 1985.
- 44, ueftier 0, Hess am J: Rerinata pulmonary prostag andin production. Am J Pnys 01 241:756-759, 1981.
- Starling M, Nautze J, Eliio~ H: Dontmol of elavaten pulmonary vascular ras stande in neonatal sw na w th prostacyc na (P01:1. Prostagiano n Mao 3:105-117, 1973.
- Lay n DL, Weinberg AG, Rerkin HM: Pulmonary micmotninombi synoroma in newborn infants w th unresponsive persistent p.. monary hyparTans on, J Ran atm 102:299-303, 1983.
- Horgan M, Demrasco N, Risembaing H: Tne relat onan p of tnrombocytopen a to the onset of persistent pulmonary hypertension of tne newborn in meconiom asprat on synorome. NY State J Med 85:245-247. 1985.
- Hemmarman 0, Less N. Strates F at al.: Prostanolos in neonates w th pars stent p~ monary nypertension. J Padietr 110:470-472, 1987.
- Hammamman 0, Komar K, Meanow 01 at al.: Select ye inhib tion of tnromboxene syntoatese ren..ces group H beta hemo ytic streptococcal-inducao pulmonary hypertension in p giats. Devel Rhermaco Them 11:306-312,1988.
- Furchgott HF, Zawadzki ..v: The obligatory role of anootneel cells n the me axation of arterial smooth muscle ny acetyonoline. Nature 288:373-376, 1980.
- St. vennootte PM: The endothelium - moduietor of smooth muac atone. N Engi J Med 319:S12-S13, 1988.
- Pmocaedngs of tne Nat one Heart, Ldng and H ond institute Pediatric Derdiology Workshop: Pulmonary Hypertension. Freomen WF fadl. Redatin Has 20:811-824,1986.
- Sn mocawa H, Aeros EL, vanho..tta PM: Porcine coronary arteries wth regenerated endothalium have a reouceo anootnalium dependent responsiveness to eggragat ng plate eta and saroton n. Dm0 Has 61:256-270,1987.
- Ogewa 5, Garlaco H, Esposito 0 eta.: Hypox a modulates toe barmier ann coag.. ant function of cuitured boy na endotoeliom (increased mono eyar parmean ty and induction of procoagulant propart esl. J 0 n invest 8S:1090-1098, 1990.
- James ES, Apgar v: Resuscitation proceoures in the delivery room. N: Resuscitation of the Newborn Infant. St. Loois, MO, cv Mosoy Do., pp 149-176, 1960.
- Gregory SA, Goocing DA, Rn nba at al.: Macon ..m aspiration in infants: A prospective study. J Padiatr 8S:848-852, 1974.
- S7. Ting P. Drany JR: Tradnee suction in maconium aspiration. Am J Onstet Gynacol 122:767-771, 197S.
- Damson HS, Losay FvW, Howes WA et al.: Dombined obstetric ann pad atm c approach to prevent macon ..m asp rat on synomoma. Am J Obstet Gynecol 126:712-71S. 1976.
- Darson HS, Howes WA: Prevention of maconium asp rat on. Am J Obstat Gynaco 133:934, 1979.
- Hancalami F, Rem n J: Macon ..m aspiration ann other asphyxiel 0 sordars. Dun Par natol 5:317-335, 1978.
- Wiswali TE, T..ggia JM, Turner HS: Meconiom asprat on syndrome: Have we made a nifferance? Pen atinca 85:71S-721, 1990.
- Domm ttae on Fat..s end Newborn: Standards and Recommendations for Hospital Dame of Newborn Intents. 66th Ed. Evanston, IL. Am Aced Pediatrics, pp 52, 54-55. 1977.
- Hobos ..F, Edeiman AL: Meconium asp raton synorome. IN: 0 nca Management of Motners and Newborns. Marks SF (adi. New York, Springar-varag, pp. 137-151, 1979.
- Mais PJ, He M, Mamshe.i uR at a.: Macon ..m passage: A new diassif cation for rise management o..rng abor. Am J Obstet Gynacol 130:S09-S13. 1978.
- Hagaman,SR, Donlay M. Francis K at al.: Delivery room management of meconium stan ng of amniot c fluin ann the development of maconium aspration syndrome. J Rem natol 7:127-131, 1988.
- Davis HO, Philips ..B, Harris HA at el.: Fete macon um aspiration syndrome annum ng neap ta airway management cons dared appropriate. Am.. Obstet Gynacol 151:731-736, 198S.
- Sepkowitz 5: mt ..~enca of the agel mparat:va ann man cal guide nes on the indinanca and management of the maconium stained newborn. Am J Dis Dhiid 141:1124-1127, 1987.
- L nder N, Aranoa ±1, Ts..r M at el.: Need for endotmedneel ntubation end suction in meconium ste ned neonates. Peniat: 112:613-615, 1988.
- Dunningnam AS, Lawson FE, Martin RJ, Runes RS: Trachea auction ann macon um: A proposed stennerd of care. u. Pan etr 116:153-154, 1990.
- Meyrick HO. Perkatt EA: The sequence of ca .. ar and hamonynemic changes of cnronic p.. monary nypertension induced ny nypoxia ann otner stimuli. Am Rev Rasp Dis 140:1486-1489, 1989.
- Parkeft LA, Drignam KE, Meyrice H Omanumocyte nepietion attenuates sustained pulmonary nypertension ann ncreasad pulmonary vesoreect vity demised by cont n..ous air embo zation in sneap. Am Rev Respir Dis 141 :456-465. 1990.
- Packham GJ, Fox WW: Rnysioiogic factors affect ng pulmonary artery pressure in infants with persIstent pulmonary hypertension. Redatin 93:1005, 1978.
- Fox WW, Duera 5: Persistent pulmonary nypertension in tna neonate: Diagnosis ano management. J Pediatin 103:SOS, 1983.
- Ko obow T. Moratti MR. Fumaga 8 at al.: Severe mpa inmant of long function induced by hign peak airway pressure or ng macnanicel ventilation. Am Rev Rasp Dis 135:31 2-31S, 1987.
- Dora M, Koonow T, Spat ole H at el.: Severe acute rasp matory failure managed w Tb cont n..oos pos five a inway pressure and part al ext racorporee ceroon d ox da remove, ny en ertifcie membrane long. Am Rev Rasp Dis 138:1480-1487. 1988.
- Drommond WH, Gregory GA, Heymann MA at a .: The .ndependent effects of nypervant at on, tolezolina, and dopemna on intents witn pars stent pulmonary hypertension. J Pan atm 98:603-611, 1981.
- Hemnnaum JD, Russell P. Sham aen PH at al.: Long-term follow-up of newborns witn pars stent puimonary hypertension. Omit Dare Med 12:S79-S83, 1984.
- Neding JH: Historical contmo a for axtracomporee membrane oxygenat on n neonates. Dint Dare Man 17:423-42S, 1989.
- Hegaman JH, Adams MA, Gamonar TH: P.. monamy compicatons of hyperventilation therapy for persistent pulmonary hypertension. Omit Dare Mad 13:1013-1014, 198S.
- KeTy SS, Schm dt, OF: The effects of act ye ann pass ye nypervent ation on careoral 0 nod flow, oxygen cons~.mpTion, cardiac Output ano Olood pressure of normal young man. JO n invest 2S:107-119, 1946.
- Hansen NH, Nowcki PT, Miller HR at a .: Alterations n cerebra bloon flow and oxygen consumpt on o..m ng pro ongec nypocarbie. Pedetin Has 20:147-iSO, 1986.
- Harp JH, Wo man H: Derabra metabolic effects of hyperventilation ann deliberate hypotension. Drit ,. Aneestn 4S:2S6-262, 1973.
- Farrare B, Johnson DL, Oheng RN at al.: Efficacy and neurologic o..tcoma of protound hypocapneic aikalosis for tna treatment ot pars stent p.. monamy nypertension n intancy. u Padiatin 1OS:4S7-461, 1984.
- Se EJ, Gaines JA, 0 ..ckmen 0, W ems EA: Persistent fatal c inculation: Neurodeve opmentai outcome. Am J Dis On d 139:2S-28, 198S.
- Handrices-Munos KD. Walton ..P: Hearing oss in infants witn persistent fatal c inculation. Pediatrics 81 :650-656, 1988.
- B fano EM. Pfannanstiel A: Duration of hyperventilation end outcome .n infants witn persistent p.. monamy nypertension. Peolet rids 81:657-661,1988.
- Schre her MD, Heymann MA, So far SJ: increasan artaria pH, not decreased PeDO> atten..etas hypoxia- no..cao pulmonary vasoconstriction in newborn amos. Pediatr Has 20:113-117, 1986.
- Wung, J-T, James ES: Optimizing convent one reap matory s..pport. IN: Extracompomee Membrane Oxygenaton. Domnian Dana Arenaman H ledal, 1993.
- Froase AR, Dryan AD: Effects of anesthes,e and paralysis on o ephregmeT,c macnenics in man. Aneatnesiolagy 41:242-2SS, 1974.
- Ooatzmen DW, Sunshna P, Johnson ..D at ai.: Neonatal hypax a and po macamy vesospesm: Response to to azoline. Pen atm 89:617-621, 1976.
- Stevenson DK, Kasting DS, Darneil HA at al.: Refractory hypoxem,e assoc atad w,th neonatal pulmonary o sease. The ..sa and im tetions otto aza na. J Paoiatr 9S:S9S-S99, 1979.
- Stevens DO, Scomeinem Hi. Hull MJ at al.: An analysis of toazo ne tnarepy in tne din tidally iii neonate. Peo atm S~rg 13:964-970, 1980.
- Haros SJ: var at on n inspiratomy-axpimetory mat a and a inway pressure wave form during machen ce ventilation: The significance of mean a inwey prass..ra. J Peo atm 94:114-117, 1979.
- Wung J-T, James .5. K chevaKy E, James F: Management of nfents w th severe respiratory failure and persistence of the fete dm0.. at on, without nyperventilat ion. Pedatros 76:488-494, 198S.
- Dwaratz AR, Maya FR, Sane H at el.: Survive of intents witn persistent pulmonary nypartans on w thout extracorpomeal membrane oxygenation. Pediatrics 84:1-6, 1989.
- Drown H, P cker ng Di Persistent trans tional c,mcuiation. Aron Ha Ohilo 49:883-883, 1974.
- Shenearen 5, Farooki TO, Hess H: H-Hemoiytic streptococcal nfection appearing as pars stent fatal circo at on. Am J His Ohilo 136:72S-727, 1982.
- Hart,att RH, Ha off OW, Dome, HG at a.: Extrecnrpomea dimc..iet,on n neonata reap ratary fa ..ma: A prospective manoom sad study. Pediatrics 76:479-487, 1988.
- Snort DL, Miller MK, Anderson KO: Ext racarporeal mainorana axygenat on n the management of respiratory failure n tne newborn. Olin Parineto 14:737-748, 1987.
- Orts RM, 0 ray HF, Hartiett RH: Extracompomeel mamomana oxyganet on in paniatr c respiratory fa ..ra. Radiat 0 n Nortn Am 34:39-46, 1987.
- ORourka PR, Drone HK, vacanti JR at a.: Extracorporeal membrane oxygenation and convent one man cal therapy in neonates w th persistent p~. manamy hypartens on at the newborn: A prospective randomized stony. Pediatrics 84:9S7-963, 1989.
- Memron M-J, Orisafi MA, Drisco, JM at a,.: Hear ng end naurodeve opmantai outcome n survivors of persistent pulmonary nypertension of tna neonate. Redatin ca 90:392, 1992.
- Persistent twin dm0.. at on and extracompomee membrane oxygenaton. The Eancat (En tomail 2:1289-1291, 1988.
- Greenfielo U, Enart PA, Hanson DW: Effect of gas tiva pmess..re ventilation on surface tension prapert as at ung extracts. Aneatnesiology 25:312-316, 1964.
- Hamach ,~, Dirbame 0, Eggars OWN at a.: Positive pressure as a cause of respiratory induced ..ng a sease (enstracti. Ann intern Med 72:810, 1970.
- Kolobaw, T: The lirire evanca at snort term stuales. Intamneti J of Art I Organs lEditomie 113:1-2,1990.
- Scnapira H, Solimana A: is axtracomporee mamomene oxygenation necessary to meauca mortality and mombloity n pat ants witn meconium aspiration syndrome? Pedietin Has 23:424~, 1988.
- Doe OH, J son F, Kessler H: FOMO: Hegionai eve ..etion of need ann app cenity of selection cm tam a. Am J His On a 142:1320-1324, 1988.
Neurological Outcome After Extracorporeal
Membrane Oxygenation Therapy in the Newborn
Jan Goddard-Finegold, M.D.
Associate Professor of Pediatrics and Pathology
Division of Pediatric Neurology
Baylor College of Medicine and The Texas Children's Hospital
Abstract
Extracorporeal membrane oxygenation (ECMO) has been used in over 3,500 neonates with severe respiratory failure since the 1970's. Although the neurologic morbidity of the procedure has not been fully evaluated, the permanent ligation of the right common carotid artery and jugular vein and the systemic heparinization that usually accompany ECMO have been felt by many neonatal physicians to be associated with unacceptable neurologic risk. The true neurologic risk has been difficult to assess, because studies of the efficacy of ECMO to date have been difficult to control. Further application of ECMO for the treatment of severe respiratory failure in newborn infants is being questioned because of the technical complexity of the procedure, its labor intensiveness and expense, and recent reports of increasing survival of infants after updated conventional medical therapy.
In this report the mortality and neurological morbidity from published studies, the types of neurological handicaps that have been documented in survivors of ECMO and conventional medical therapy, new cerebral hemodynamic data in post-ECMO patients, and results of neuroimaging and neuropathology studies are presented. In addition, some suggestions for future studies are offered.
Survival Statistics
Extracorporeal membrane oxygenation (ECMO) has been used as a last effort treatment strategy in over 60 centers and in more that 3,500 newborns since the mid-1970's for term or near-term neonates in severe respiratory failure.2 Mortalities for conventional medical therapy (CMT) and for ECMO can be derived from published data,3-23 but only two prospectively designed studies compare ECMO to conventional treatment modalities.4,11 It has been difficult to design such studies because conventional treatment options for severe respiratory failure in newborns historically produced dismal results which were the impetus for ECMO trials in the 1970's. Thus, the major problems apparent in the studies of ECMO include variations in patient selection criteria in different centers and patient selection bias leading to inappropriate or small numbers of controls, or use of historical controls, and only modified randomized prospective studies comparing ECMO to conventional medical therapy from the mid to late 1980's.
Mortality statistics of conventional therapies, ECMO, and, in one study, high frequency oscillatory ventilation plus ECMO, available from reports published between 1984 and January 1990, are shown in Table 1. As can be seen in the table, mortality with conventional therapy has ranged from 100 percent (no survivors) to 0 percent (100 percent survivors). During the same period, the mortality with ECMO has ranged from 45 percent (55 percent survivors) to 0 percent (100 percent survivors). The survival for conventionally-treated infants in these studies was 126/185 (68 percent) and for ECMO-treated infants 377/470, (80 percent). These compiled figures compare with the Neonatal ECMO Registry's survival statistics as of January 1990–83 percent for ECMO-treated infants.1
Table 1: Survival Statistics
Abbreviations:
CMT—conventional medical therapy, HFOV—high frequency oscillatory ventilation, ECMO—extracorporeal membrane oxygenation therapy.
* All ECMO patients also had HFO trial first and failed.
+ CMT=HFOV.
# Retrospective study of outcome during two time periods; the 1986—1988 period is used here.
** Two patients had major congenital anomalies.
++ Phases 2 and 3 of a 3-phase study.
## "Play-the-Winner" strategy.
*** Results represent newborns only; study also included some children treated with ECMO.
| Year | Author | Percent ECMO Survival | Number of Infants | Percent CMT Survival | Number of Infants |
|---|---|---|---|---|---|
| 1990 | Carter | 88* | 22/25 | 100+ | 21/21 |
| 1990 | Adolph | 80 | 101/126 | - | - |
| 1989 | O'Rourke | 97 | 28/29 | 60 | 6/10 |
| 1989 | Dworetz | - | - | 89# | 8/9 |
| 1989 | Nading | - | - | 100# | 5/5 |
| 1989 | Glass | 84 | 84/100 | - | - |
| 1988 | Bifano | - | - | 72 | 26/36 |
| 1988 | John | - | - | 88 | 24/27 |
| 1988 | Ortega | - | - | 57 | 21/37 |
| 1987 | Redmond | 79 | 42/53 | - | - |
| 1986 | Andrews | 58** | 14/24 | - | - |
| 1986 | Bartlett | 90++ | 45/50 | - | - |
| 1985 | Bartlett | 100## | 11/11 | 0 | 0/1 |
| 1985 | Towne | 55*** | 24/43 | - | - |
| 1984 | Ferrara | - | - | 88 | 18/23 |
| 1984 | Krummel | 67 | 6/9 | - | - |
| 1984 | Bernbaum | - | - | 49 | 18/37 |
When the reported numbers of surviving infants for ECMO and CMT from the studies published between 1984 and 1987 are compared, the survival is 142/198 (74 percent) for ECMO and 36/61 (59 percent) for CMT. Between 1988 and 1990, these figures change to ECMO survival of 235/280 (83 percent) and CMT survival of 90/124 (74 percent) with three retrospective studies of CMT reporting survival of 88 percent–100 percent.5,17,22 These studies are likely to reflect results to come, because modification of conventional therapy has been occurring over the past 10 years in centers whose neonatologists have been unwilling to risk carotid and jugular ligations for ECMO therapy in their young patients, but who have recognized the serious limitations of previous conventional therapy comprised mainly of pulmonary hyperventilation.
Non-or inadequately-randomized studies of ECMO have been justified in the past by mortality in conventionally treated infants close to 80 percent. Recent evidence indicates that mortality in these infants may now be realistically as low as 0–10 percent in some centers. Thus, ideally, there is currently no justification for designing studies that are not controlled and that do not have truly randomized population samples. Practially and ethically, however, it would be extremely difficult to carry out such studies without offering ECMO as "rescue" therapy for the small number of infants failing the most up-to-date conventional therapies.
Neurological Morbidity Statistics
Obviously, of greatest concern to those setting criteria for and instituting ECMO and other types of therapy is the ultimate neurodevelopmental outcome of surviving infants. The necessity for right carotid arterial cannulation and ligation and for systemic anticoagulation during ECMO add potentially increased risks of ischemia and/or hemorrhage to infants already subjected to hypoxia/asphyxia and hypotension from cardiorespiratory compromise.
There are several neurodevelopmental follow-up studies of children treated with conventional therapy for persistent pulmonary hypertension and other causes of severe respiratory failure18-24 A compilation of results from studies published since 1984 is shown in Table 2. Neurologic morbidity has ranged from 11–30 percent and is as high as 79 percent if sensorineural deafness is included as a neurologic abnormality18,19 Developmental testing has revealed definite abnormalities in 0–18 percent and suspected delays in from 0–43 percent.
Table 2: Neurodevelopmental Morbidity—CMT
Abbreviations:
F/S—followed/survived, F/U—follow-up period, NL—normal, SUSP—suspect, ABN—abnormal.
*Calculated from data presented.
+Exclusive of hearing loss.
| Date | Author | F/S | F/U Period | Assessments | Percent NL | Percent SUSP | Percent ABN |
|---|---|---|---|---|---|---|---|
| 1988 | Munoz | 40/50 | 1 yr | Audiologic Neurologic Bayley |
48 72 100 |
- - - |
52 27 0 |
| 1988 | John | 23/24 | 3 yr | Bayley or McCarthy | 60 | 8 | 30 |
| 1988 | Bifano | 21/26 | 1 yr | Neurologic Bayley |
53 38 |
28 43 |
19 19 |
| 1985 | Sell | 40/40 | 4 yr | Audiologic Neurologic* Bayley or McCarthy |
80 40 92 |
- 22 - |
20 18+ 8 |
| 1984 | Bernbaum | 11/18 | 4 yr | Neurologic Bayley or Stanford-Binet |
72 81 |
- - |
27 18 |
| 1984 | Ballard | 9/11 | 5 yr | Neurologic McCarthy; Stanford-Binet |
88 88 |
- - |
11 11 |
| 1984 | Ferrara | 16/23 | 1 yr | Neurologic Bayley |
87 100 |
- - |
13 - |
Follow-up of ECMO treated infants has been reported in eight major studies to date.2,6,7,9,12,13,25,26 (The results from Redmond et al. are included in the mortality statistics but not in the follow-up statistics.8 This paper reported neurologic abnormalities in 3 of 42 survivors, but no developmental testing or neurologic exam results were reported for specific follow-up periods). Data from the studies in Table 3 are from only those patients treated with ECMO as newborns. Follow-up periods have now ranged from 1 to 10 years. Most studies have included combined neurodevelopmental assessments and have found abnormalities in motor development in 2—35 percent. Abnormalities determined by neurologic examination alone have been found in 2–38 percent. Abnormalities in mental indices have been present in 0–28 percent. Severe combined disabilities have been present in 2—10 percent.
Table 3: Neurodevelopmental Morbidity—ECMO
Abbreviations:
F/S—followed/survived, F/U—follow-up period, NE—normal, SUSP—suspect, ARN—abnormal.
*These 42 patients of Glass at al. are a subset of the 71 reported by Taylor at al. (#6) and are not used for derivation of numbers of patients with morbidity on developmental exam. Because results of the neurologic exam are not reported in #6, the neurologic morbidity from Glass at al. has been used for derivation of neurologic morbidity.
| Date | Author | F/S | F/U Period | Assessments | Percent NL | Percent SUSP | Percent ABN |
|---|---|---|---|---|---|---|---|
| 1990 | Adolph | 57/90 | 4 yr | Neurologic | 98 | - | 2 |
| Bayley, McCarthy, Gesell, DDST | 79 | 19 | 2 | ||||
| 1989 | Glass* | 42/46 | 1 yr | Neurologic Bayley |
75 59 |
19 19 |
5 21 |
| 1989 | Taylor | 71/90 | >1 yr | Bayley | 61 | 17 | 23 |
| 1986 | Andrews | 14/14 | 3 yr | Bayley (motor) (mental) |
64 71 |
- - |
35 28 |
| 1985 | Towne | 18/24 | 8 yr | Neurologic Standford-Binet | 62 | - | 28 |
| 1984 | Krummel | 6/6 | 21 mos | Neurologic Bayley |
83 100 |
- - |
17 0 |
Although the degree of long-term motor and cognitive impairment cannot be determined with certainty until school age, the number of severely neurodevelopmentally handicapped children after ECMO appears to be low. Unfortunately, most follow-up studies to date have not included conventionally treated, age, socioeconomicallymatched, or sibling controls in order to determine what proportion of the deficits are directly related to treatment modalities. This has begun to change, however, as long-term follow-up studies including at least some matched control subjects are now beginning to be published.26 In the past it has been difficult to make interinstitutional comparisons of outcomes, or to make sense of CMT and ECMO reports from patients treated in two essentially different eras. This has also become less of a problem, as procedures and criteria for therapy have become more standardized.
Types of Neurological Handicaps Documented in Survivors
The neurological abnormalities reported in children after CMT have included hearing loss, moderate gross motor delay, fine motor delay, static encephalopathy, hydrocephalus plus cerebral atrophy, speech impairment with and without hearing loss, and severe, global motor and mental retardation.18-24
Similarly, the predominant neurological handicaps observed in survivors of ECMO have included motor delay, mental delay (including some with severe, global delay in both areas), static encephalopathy, and expressive language disorders.2,6,7,9,12,13,25,26 Recently, hearing loss has also been documented in 4 percent, 8 percent, 21 percent, and 23 percent of ECMO survivors tested audiologically in four centers.14,15,25,26
Results from a recent survey of follow-up information from ECMO centers have been summarized by Glass.27 Most post-ECMO infants show abnormal tone and reflexes for up to 1 month after ECMO. Upon discharge, parents note difficulties with oral feeding and frequently must use gavage feeding for a period of time. By 4 months postnatal age, 25 percent of ECMO babies have hypotonia, mild asymmetry, and/or mild motor delay, and head control is usually delayed.27 By 1 year of age, the significant handicap rate reported in the survey has been from 0—20 percent. This impairment rate has been consistent for infants followed until 2 years of age. However, by 2 years of age, Glass notes that 25—30 percent are showing deficits in language or perceptual skills. Visual-motor abnormalities have been detected in 11—21 percent at 3 years of age.27
Both postneonatal seizures and hemipareses have been infrequently reported, although a recent retrospective analysis of a subset of patients in an ECMO treatment group (the data from which have not been fully analyzed) has suggested that left lateralizing neuromotor abnormalities, indicating damage to the right cerebral hemisphere, are more frequent than previously recognized.28 This study by Schumacher et al. reported 8 of 59 ECMO survivors; of the 8, 3 infants had exclusively left-sided upper and lower limb hypertonicity and hyperreflexia.28 Two of the eight had mild left hemipareses, and two others had bilateral findings with left sided predominance. One patient had spastic quadriparesis. Two of the eight patients had documented neurologic abnormalities prior to ECMO therapy, and five of the eight patients required cardiopulmonary resuscitation. Electroencephalographic (EEG) abnormalities were present in all eight patients and were right lateralizing in five. Because this report did not include statistics for the entire population of survivors, it has not been included in the mortality or morbidity statistics in Tables 1, 2, and 3. Another study, by Campbell et al.,9 reported an increased incidence of right cerebral hemisphere seizure activity after the initiation of ECMO; however, there was no significant right hemispheric lateralization in patients with hemipareses, atrophy, or EEG abnormalities. The study only documented immediate post-ECMO neurologic findings, and, thus, is not included in the tables.
Follow-up EEG studies have been done in 10 of the 18 patients first reported in 1984 by Towne et al.12,30 EEG's in these patients at 4 to 9 years of age have revealed abnormalities in three; only one had evidence of a focal abnormality in the right hemisphere, and none of the patients had clinical postneonatal seizures.30
Other neurophysiological studies in these patients included auditory and somatosensory evoked potentials. The only significant difference found between the controls and the post-ECMO patients was in hemispheric amplitude symmetry for long-latency auditory and somatosensory evoked potentials.30 This may imply that there is a decrease in responsiveness of the right hemisphere compared to the left after contralateral input; as the authors point out, however, it may also indicate a change in the orientation of the dipole generating the potentials and not be a reflection of hemispheric responsiveness. It has been suggested that the amplitude alteration in the cortical component of the evoked potential measures are related to persistent low cerebral blood flow in the right carotid system.
One study reporting acute neurological problems associated with ECMO, and another reporting a small subset of patients culled from a larger population, suggests that right hemispheric lesions may be more common than previously recognized; this conclusion is not supported by the follow-up studies to date. More information is needed, including results of assessments of motoric skills and higher cognitive functions (including evidence for "right hemisphere syndromes") in older children who have survived ECMO.
Hemodynamic Evaluations of Survivors of ECMO Patients
Hemodynamic evaluations of ECMO patients are being reported with greater frequency.30,33 Follow-up studies of the patients first reported by Towne et al.12 have included evaluations by Doppler ultrasound for carotid blood flow velocity estimates, in addition to the EEG and evoked potential studies mentioned earlier.30 Nine of the patients had permanent ligation of the right common carotid; one had axillary artery ligation. The estimated timed average velocities in the right internal carotid arteries were about 62 percent of those on the left, and the diameter of the right-sided vessel averaged 3.8mm compared with 5.8mm on the left. Flow velocity in the right carotid was 26 percent of that in the left. In 15 patients without carotid ligations, there were no differences in timed average velocities of flows between the left and right sides. 30
Velocity and direction of blood flow in vessels of the circle of Willis were assessed in three infants before, during, and, in two of the three, after ECMO in one study.31 Fifteen minutes following carotid ligation, the maximum spectral velocity and timed-average mean blood flow velocity were reduced 37 to 50 percent in the right middle and anterior cerebral arteries compared to the pre-ECMO values in one infant. In the other two infants right middle and anterior cerebral artery velocities were either normal or reduced, and in one of the infants velocity was increased 116 to 217 percent on the left side. Flow direction was reversed in all three of the infants in the A1 segment of the right anterior cerebral artery and in the right posterior communicating artery, suggesting provision of right middle cerebral arterial perfusion via the vertebrobasilar and contralateral carotid systems through the circle of Willis.
Lewin et al. have reported results of magnetic resonance (MR) angiography in 15 neonates after ECMO and right common carotid ligation (RCCL); in four 1-year old infants after ECMO with RCCL, and in four neonates after ECMO following right common carotid artery reanastomosis.32 The MR angiography was satisfactory in 20 of the 23 patients, and, in 9 of 16 with permanent RCCL, as well as in all patients with right carotid reanastomosis, the right internal carotid artery was patent proximal to the ophthalmic artery. In the six nonreanastomosed neonates with internal carotid patency, signal intensity in the right proximal internal carotid was lower than in the left. In all of the reanastomosed neonates, patient right internal carotid arteries were documented, although signal intensity was reduced in one.
Taylor et al. have reported increases in mean cerebral blood flow velocity up to 87 percent during ECMO in 73 percent of patients. This has been accompanied by decreased pulsatility of flow.33 Vasodilatation and increased cerebral blood flow are responses to the relatively high PCO2's that are maintained to sustain ventilatory drive during ECMO.33 In newborn lambs, ECMO initiation during asphyxia caused severe decreases in intracranial pressure combined with increased carotid arterial blood flow (as in other newborn animals, this augmentation in blood flow was also seen with asphyxia alone).34 The reason for decreased intracranial pressure in conjunction with increased cerebral blood flow in this experimental situation is not yet clear.
The compensatory responses to RCCL are not the same from patient to patient; however, flow velocity to the right hemisphere is lower than normal and lower than that in the left hemisphere in most of those evaluated by Doppler or MR angiography, indicating that compensation via the circle of Willis does not restore flow completely. Clinical ramifications of these findings include (1) the possibility of greater right hemispheric vulnerability later in life, when patency of the vessels on the left is compromised by atherosclerosis, and (2), the possibility that suboptimal blood flow to the right side, if not responsible for major deficits, may nevertheless be associated with subtle metabolic derangements that affect cellular function. Screening of circle of Willis anatomy and responses to transient common carotid occlusion prior to ECMO would probably be useful in patients stable enough for testing, as anatomical studies of human fetuses suggest that a complete and symmetrical circle of Willis is present in only about 21 percent.31,35
Neuroimaging
Neuroimaging has been carried out in most patients before, during, or after ECMO.35-40 Table 4 is a compilation of reported sonographic and computerized tomography (CT) findings. The importance of the pre-ECMO ultrasound (US) and the post-ECMO CT scan becomes evident from this information. Of the 297 patients reported in four studies, 127 had brain abnormalities documented by US; in 2 of the studies abnormalities prior to ECMO were documented in 40 of 257 patients.37, 39 CT scans performed 1—2 weeks after discontinuation of ECMO showed abnormalities not detected by US in 42 of the 257 patients. When compared with autopsy findings, US abnormalities were frequently non-specific, i.e., areas of abnormal brain appeared echogenic with or without blood, and partially clotted blood showed a wide range of variation in echogenicity.37 CT scans usually detected cortical infarots, atrophy, peripheral hemorrhages, petechiae, and low density lesions that were not detected by US. Correlation of neuroimaging results with developmental follow-up by Taylor7 showed that 80 percent of infants with normal US and CT scans were neurologically normal on follow-up; 70 percent of infants with mild abnormalities on US and CT were normal, and 20 percent with moderate abnormalities were normal. None of the infants with severe abnormalities on US and/ or CT was neurologically normal.7
While the ultrasound examination is useful and easy to accomplish at the bedside, it may be neither specific nor sensitive in this population of patients. Ideally, one pre-ECMO CT scan should be obtained; obviously, this is not always practical. Therefore, at least one post-ECMO CT should be obtained in each survivor within the first month after discontinuation of ECMO.
Neuropathology
Neuropathology has been correlated with neuroimaging findings in 17 infants.35 Characteristic neuropathology of ECMO-treated patients includes multifocal white matter infarcts, parenchymal hemorrhages with parenchymal necrosis, germinal matrix and intraventricular hemorrhages (usually in preterm infants), and cystic lesions and gliosis.35 Infarots associated with aluminum emboli have also been described.36 Edema is frequently present prior to ECMO and can occur during ECMO. Microscopic abnormalities have included neuronal necrosis, microcalcification, hemorrhage, and gliosis.37 Gliosis and calcification are late stages of cerebral infarction evident about 5 days after injury.
Parenchymal hemorrhage, cerebral edema, and white matter and cortical infarction are common in patients with hypoxic-ischemic encephalopathy and probably reflect the degree to which all of these ECMO-treated infants have been hypoxic and hypotensive. However, the systemic heparinization of ECMO-treated infants exacerbates bleeding that frequently occurs during reperfusion in areas that have been ischemic. Ischemia and hemorrhage may also be exacerbated during ECMO, despite increased oxygenation, by the acute changes in cerebral blood flow, pulsatility, and intracranial pressure associated with ECMO itself (and not solely with carotid ligation).
Although systemic heparinization during ECMO contributes to increased incidence and severity of cerebral hemorrhage, and although iatrogenic complications of ECMO can include aluminum cerebral emboli, most of the neuropathology closely resembles that of hypoxicischemic encephalopathy.
Prediction of Outcome
Prediction of outcome prior to ECMO therapy has been difficult except in patients with large hemorrhagic lesions already apparent on ultrasound.40 Most infants have been difficult to evaluate neurologically prior to ECMO; many have received anticonvulsant or paralyzing drugs, and all have been severely hypoxic. Many have been supported with vasopressors, and some have been septic. One study has attempted to correlate neurological morbidity with pre- and post ECMO factors.6 In this study by Glass et al. four factors were related to neurological morbidity at 12 months of age: sepsis on admission was the most highly correlated; chronic lung disease (defined as a supplemental oxygen requirement for ³ 1 month after ECMO) was next highly correlated; an intracranial abnormality on US or CT was third, and lower gestational age was borderline. To date, seizures before or during ECMO have not been reported as predictive of outcome. Recently, performance on a visual attention task (P-VAT) at 1-month postnatal age has correlated with neurodevelopmental outcome at 1 year of age.27
Where Do We Go From Here?
Undoubtedly, as new technologies become available, the options for treatment of these infants will increase. The availability of other ventilatory therapies that reduce barotrauma, the possibility of carotid reanastomosis, the use of double-lumen catheters for veno-venous ECMO, and provision of heparin-lined tubes that obviate the need for systemic heparinization are just some of the advances that can be foreseen for this population of severely-ill infants. In addition, new bedside methods for evaluation of cerebral blood flow and metabolic changes, such as near infrared spectroscopy, will make it easier to assess the status of the central nervous system and perhaps provide more meaningful indices for prediction of neurologic outcome.
In the meantime, all ECMO programs and all ECMO studies should include neurodevelopmental follow-up of patients. Such follow-up requires, in addition to neurologic and developmental assessments, neuroimaging and sophisticated neurophysiological studies. Instruments for evaluation of cognitive and motor abilities should be similar at each follow-up center and appropriate for postnatal age. Children should be followed long enough to be able to assess fixed motor deficits and higher cortical functions (school age or beyond). It is also imperative to prospectively assign sibling controls and controls for variables such as degree of prematurity, age, sex, and socioeconomic status. Previous studies have neglected these elements of properly designed follow-up, reducing the value of their findings considerably.
Goals for the future should be (1) to standardize ECMO treatment and follow-up programs and (2) to assess neurodevelopmental status as completely as possible prior to, during, and after ECMO. To be meaningful, such programs require (1) standardization of criteria for treatment and exclusion; (2) standardization of ECMO and CMT procedures; (3) a mechanism for long-term follow-up to assess neurologic outcome, developmental indices, and neurophysiologic measures well into school age; and (4) inclusion at follow-up of a well-designed control group matched at least for gestational age, sex, and socioeconomic factors. It would also be ideal to be able to include as a separate control group in follow-up those infants who are "near-miss" ECMO candidates.
Acknowledgments
Supported in part by the National Institute of Neurological and Communicative Disorders and Stroke.
References
- EDMO Registry. Ann Arbor, Mi, 1990.
- Anoiph v, Ekelund 0, Smith 0 eta.: Dave apmentai outcome at neonates treatan witn axtracorporeal mamarane oxygenation. J Padiatin Suing 2S:43-46, 1990.
- Darter JM, Gemstmann DR. Dark RH at al.: High-fraguency nec atory ventilation and extracompamea membrane oxygenation for faa treatment of acute neonatal respiratory failure. Red atinca 85:159-164, 1990.
- OHourKe PP. Drone RK, vacant JR at al.: Extracarpomeal mamamene axygenat on and conventional medical therapy n neonates with pars stent pulmonary nypertansion of tna newborn: A prospective manoomizan study. Pad atm ca 84:9S7-963, 1989.
- Dworetz AR, Maya FR, Saba H at al.: Somvivai of nfants w th pars stent p.. monamy hypertension without extracompomas membrane axyganatan. Ran atinca 84:1-6, 1989.
- Glass P. Miller H. Short H: Morbidity far survivors of extracomporee membrane oxygenation: Neumodeve opmantai outcome at 1 year of age. Renatin ca 83:72-78, 1989.
- Tay or GA, Fits OH, Glass P at al.: OT of camanmovescuiam niumy after neonatal extmacamparaai membrane oxygenation: implications for ne..ronavaiopmenta outcome. Am J Radial 1S3:121-126, 1989.
- Heamano OH, Graves ED, Faiterman KW at al.: Extracomporae membrane oxygenation tam respiratory failure and cardiac tailume n infants and children. J Tanrec Dardioveac Suing 93:199-204, 1987.
- Andrews AF, N xan DA, Dilley RE at al.: One- to three-year outcome tom 14 s.~mvivoms of extracompomeal membrane axygenat an. Pediatrics 78:692-698, 1986.
- Bartlett RN, Toomasan J, Rolaff Data.: Extracarpareal membrane oxygenation (EDMO) n neonatal respiratory ta ore: 100 Oases. Ann Suing 204:236-24S, 1986.
- Hartiett RN, Roloft DW, Dorneil HG at al.: Extracompomes o inculation in neonate respiratory tailuma: A prospective random zed study. Pediatrics 76:479-487, 198S.
- Tawne RH, uott IT, HicKs HA at al.: Long-term fo ow-up of infants ann chilamen treated wth axtracorporee membrane oxygenation (FOMO): A preminary report. J Ran atm Suing 20:410-414, 1986.
- Kmummai TM, Graanfieo U. Kinapatrick Dv at a,.: The early evaluation of survivors after axtracompamaa membrane oxygenation for neonate pulmonary fa..~ma. J Padietin Suing 19:S8S-S90, 1984.
- Epsi K. Glass P, Short H: Presence of hearing loss in children following EDMO therapy: A follow-up stuoy at 2 years of age. Reolatin Has 27:247A, 1990.
- Hofkosn H. Olouse H. Smita-Janas Jets.: Ten years of EDMO: Naumodeva apmentai outcome among survivors. Peniatin Has 27:245. 1990.
- Ortega M. Hamas AD. Patzkem ADO at al.: Early predict on of ultmata outcome a newborn infants with severe respiratory failure. J Pediatin 113:744-747. 1988.
- Nading JH: Histamica controls far extracampameal membrane oxyganat an n neonates. Omit Dame Mad 17:423-42S. 1989.
- Hendmices-Munoz KD, Walton JR: Hearing 055 in infants with persistent fetal circulation. Pediatrics 81:650-656, 1988.
- Se EJ. Genes JA, Gluckman 0 at al.: Persistent fetal c mcuietion—neumodevelapmantai outcome. Am J His On d 139:25-28, 198S.
- Dernbe~m JO. Husse P. Sherioan RH at al.: Lang-term fallow-up of newborns with pars stent pulmonary hypertensian. Omit Dare Med 12:S79-S83. 1984.
- Hailard HA, Leonard OH: Developmental follow-up of nfants witn parsatent pulmonary hypertension of the newborn. Olin Rarinatol 11:737-744, 1984.
- Farmers H. Johnson OF, Oheng P-N eta.: Efficacy ano neumologc outcome of profo..ad hypocepnaic aika 055 for the treatment of persistent pulmonary hypertension in infancy. J Rediatin 105:457-461. 1984.
- Hifano FM, Pfannenstie A: Durat an at ayparventiiation end outcome a afants with persistent pulmonary hypertension. Pediatrics 81:657-661, 1988.
- John F, Roberts v, H..rnaro ED: Persistent pulmonary hypertension of tne newborn treated with hypervent at on: O a ca features and outcome. Aust Peadiatr J 24:357-361, 1988.
- Scaumacher HE. Palmer TW. Holoft DW eta.: Fallow-up at infants treated with extracompomea membrane oxygenat on for newborn respiratory failure, Pediatrics 87:451-457, 1991.
- Hofeosh H. Thompson AE. Nozza H.. at a .: Ten years of extracarpomeal membrane oxygenation: Na..mooeveiopmente outcome. Pediatrics 87:5499-555. 1991.
- Glass P. Rat ant o..tcames after neonatal EDMO. IN:
Areasman H. Dornisa H ledsl. Extmacarparaai Life Support. Oambrdge. MA. HacKwaii Rob nations, 1990. - Schomenam RE. Harks JDE. Johnston MV at a.: H ght-sided omain lesions in afants following ext racompomes membrane oxygenation. Redietin ca 82:155-161, 1988.
- Campbell ER. Dunyapen 0, Holmes GE eta.: Right common carat d artery ligation in extracorporeal membrane oxygenaton. J Rediatin 113:110-113, 1988.
- Eott IT, McPherson H, Towna H at al.: Long-term neumophysi010gb o..tcame after neonate axtrecompareal membrane oxygenation. J Radiatin 116:343-349. 1990.
- Haiu TNK. Kim SY, Mailer JE at al.: 0 india of Willis biaaa ye ac ty and flaw drection after common carat d artery gation for neonate axtmecorparea membrane oxyganat on. Pediatrics 83:343-347. 1989.
- Eawin JS. Masamyk TJ. Nadic MT at al.: Extracarpareal membrane oxygenat an in afants: Angiogmaphic and pamanchyma evaluation of tna brain with MR magiag. Radio 173:361-365. 1989.
- Tay or GA. Snort DL. Glass Pate.: Demebral hemanynamics a infants undergo ag extracomporas membrane oxygen-at an: Fort nam observations. Renal 168:163-167. 1988.
- Ste am OJH. Hayes 0: Extracorporas membrane oxygen-at an causes a gnifcaat cnangas a atracran al pressure ano carat d artery a and f ow a newborn amba. J Pedietin Suing 23:1163-1168, 1988.
- Saydal HG: Tna a amater of cerebral arteries in numan fetus. Anat Dec 150:79-86. 1964.
- Teyor GA. Snort DL, Fits OH: imaging of cemabmovascoiar aj..ry a infants treated w th extracarpomes membrane oxygenation. J Radatin 114:635-639, 1989.
- Taylor GA. Ftz OR. Kepum Sat al.: Oemabmavasc..lar accidents a neonates treated w th ext macompomee mainarana oxyganat an: Sonogmapn.c-pathoag c carrelat an. Am J Radial 153:355-361. 1989.
- Matamaros A, Anaemson JO, McDonnell.. eta.: Neumasonogmaphic finn ags a infants treated by extrecompareal membrane oxygenat on (EDMO). J On d Ne..rai 4:S52-S61. 1989.
- Haboace uS, Han BK, We as HG at a.: Drain annormalities a infants on ext racarpomeal membrane oxyganat on: Sanogmaphic and OTfindings. Am J Radio 153:571-576. 1989.
- E.. sin A, Graviss ER, WebarT at al.: Ne..masonogrepnic changes in newborns treated with extracorporeal membrane oxygenation. J tt trasauno Mao 7:429-438. 1988.
Extracorporeal Membrane Oxygenation Versus
Conventional Medical Therapy
for Management of Persistent Pulmonary
Hypertension of the Neonate:
A Decision Analysis
Craig Fleming, M.D.
Department of Medicine, Department of Community and Family Medicine, and Program in Medical Information Science, Dartmouth Medical School, White River Junction Veteran Affairs Medical Center, White River Junction, Vermont
Terry A. Hurlbut, M.D.
Program in Medical Information Science, Dartmouth Medical School
Harold C. Sox, M.D.
Department of Medicine, Dartmouth Medical School
Abstract
The use of extracorporeal membrane oxygenation (ECMO) to treat severe respiratory failure in the full-term infant with persistent pulmonary hypertension of the neonate (PPHN) has grown dramatically in recent years. Two randomized studies have established the efficacy of ECMO vs. conventional medical therapy (CMT) for severely-ill infants. But, because ECMO is invasive and resource intensive, controversy persists over the proper role for ECMO in the management of PPHN. Of particular concern is whether ECMO is more effective than CMT for the less-sick infant. We reviewed the medical literature and built a decision analysis model to compare the risks and benefits of ECMO with CMT for management of infants with PPHN. The model predicts that ECMO is the preferred therapy when the probability of death with conventional therapy is ³ 43 percent. CMT is the best choice when the expected mortality is £ 17 percent. In neonates for whom the risk of death is intermediate, the choice between ECMO and CMT is ambiguous because of imprecision in present data on the risk of long-term sequelae. To define the proper roles for ECMO and CMT in treating infants with PPHN, we need clinical prediction rules that can accurately represent differences in severity of illness for these infants and a better understanding of the impact of preventive measures and recent improvements in conventional therapy for PPHN. Meanwhile, ECMO should be used cautiously in less-sick infants.
Introduction
ECMO is a rapidly emerging technology used for management of severe respiratory failure in full-term and post-mature neonates with PPHN.1, 2 Two randomized clinical trials have proven the efficacy of ECMO in reducing mortality for severely-ill neonates.3, 4 Still, some clinicians have expressed reservations about its widespread use.5, 6 ECMO is resource intensive and requires development of local expertise to be used effectively.' Some centers have favorable outcomes with conservative management.5, 7,8 Lastly, ECMO is an invasive procedure. The most commonly used ECMO technique involves ligation of the right common carotid artery and requires systemic anticoagulation with heparin. Cerebrovascular accidents, which may cause death or lifelong disability, complicate ECMO treatment.9, 10
To understand the role of ECMO and CMT, we have reviewed the medical literature and built a decision analysis model. 11, 12 This model uses available information to compare the two therapies based on their competing risks and benefits.
Methods
PPHN is a condition that leads to respiratory failure in full-term and post-mature infants.13 Although PPHN may occur idiopathically, it is often associated with other conditions, such as meconium aspiration syndrome, congenital diaphragmatic hernia, and neonatal sepsis. The pathophysiological hallmark of PPHN is marked elevation of pulmonary artery pressure leading to large right-to-left shunts of blood flow through the foremen ovale and ductus arteriosus. Therefore, neonates with PPHN develop marked hypoxia that does not improve as the inspired oxygen concentration is increased.
The aim of therapy for PPHN is to support the neonate by maintaining adequate oxygenation while waiting for reversal of the pathophysiologic changes which underlie the syndrome. CMT relies on mechanical ventilation to achieve adequate oxygenation and may include therapy with pulmonary vasodilators, paralytic agents, and inotropic drugs.5'4 ECMO uses cardiopulmonary bypass with a modified heart-lung machine to insure adequate oxygenation while the infant recovers.2
Long-term sequelee of PPHN may arise from the underlying conditions (e.g., meconium aspiration syndrome, congenital diaphragmatic hernia or perinatal hypoxic ischemic insult) or from supportive therapy itself. These include varying degrees of neurological deficit, ranging from profound disability to mild psychomotor developmental delays, and chronic lung disease, resulting in reactive airway disease or requiring prolonged oxygen supplementation.
ECMO therapy presently requires systemic anticoagulation with heparin to prevent thrombosis of the bypass apparatus. Anticoagulation may induce or make worse hemorrhagic complications, especially intracranial hemorrhage. Therefore, ECMO is not practical for treating respiratory distress in premature infants, who are at greater risk for intracranial hemorrhages.'5 Some neonates treated with ECMO also develop non-hemorrhagic cerebral infarctions that may be related to ligation and cannulation of the right common carotid artery as part of ECMO therapy.9,10
The Decision Tree
The decision tree shown in Figure 1 models the complications and outcomes of treatment for PPHN with both ECMO and CMT. The first two complications modeled for each treatment are intracranial hemorrhage and cerebral infarction (Figure 1 A). An infant may die from either type of cerebrovascular accident (CVA), but we assume any infant may have only one type of CVA.
INSERT HERE
Figure 1. Decision tree model of the complications and outcomes of therapy with ext racorporeal membrane oxygenation or conventional medical therapy (CMT) in a term infant with persistent pulmonary hypertension of the neonate (PPHN). Panel A; the decision to treat and its immediate consequences. The square node at the left is the decision node. Circular nodes represent chance events and the rectangular nodes are health states. The diamond labelled PPHN refers to the PPHN subtree. Panel B: the subtree shows the events and terminal health states for infants with PPHN.
Table 1: Multi-Attribute Utility Score and Health States Attributes Used to Represent Quality of Life Among Suvivors of Persistent Pulmonary Hypertension of the Neonate
Key: P—physical mobility and activity; H—self care and role activity; S—emotional well-being and social activity; H—health problem.
| State | Health sate attributes | Utility |
|---|---|---|
| Healthy |
P: no limitations R: no limitations S: happy and relaxed most of the time; average number of friends and contacts H: no health problems |
1.00 |
| Chronic Lung Disease |
P: some limitation in physical ability to lift, walk, run, jump or bend R: able to care for self, but some limitations in work, school and play S: happy and relaxed most of the time; very few friends and contacts H: having a medical problem which causes discomfort for a few days each month |
0.67 |
| Moderate Disability |
P: needing assistance and mechanical aids to get around house, yard or community R: able to care for self, but some limitations in work, school and play S: happy and relaxed most of the time; very few friends and contacts H: needing special school because of difficulty in learning or remembering |
0.31 |
| Severe Disability |
P: not able to use or control arms and legs R: not able to care for self; not able to attend school or work S: anxious and depressed most of the time; very few friends and contacts H: being blind or deaf or not able to speak |
-0.39 |
Infants who survive a CVA or have no CVA enter the PPHN event subtree (Figure 1 B). In this subtree, an infant either may die of PPHN, survive in good health, or survive with 1 of 3 disability levels: severely disabled because of profound neurologic disability; moderately disabled because of a neurodevelopmental disability; or disabled from chronic lung disease. While an infant might experience several different disabling events during the clinical course depicted in the model, we simplified the model by assigning survivors to the health outcome state that represents the most serious disability. We assume this disability state will cause the greatest impairment of the child's functional capacity and quality of life. For example, a child who is profoundly disabled from an intracranial hemorrhage may also develop chronic lung disease, but profound neurologic disability will have the most impact on the quality of life. We assign survivors who have no disabilities or only mild impairments to the healthy state.
Value of Health Outcome States
The summary measure of health outcome, obtained from analysis of this decision tree, is quality-adjusted survival. By survival we mean long-term viability. We consider infants who survive initial treatment for PPHN to be non-survivors if they die in the first year or two of life from complications of therapy or underlying disease. We assign survival a value of 1 for survivors and 0 for non-survivors.
Table 2: Mortality in Studies of Persistent Pulmonary Hypertension of the Neonate Managed With Conventional Medical Therapy and Summary Mortality for Groups of Studies; 95% Cl: 95% Confidence Interval
| Study | Study Years | Selection Criteria | Neonates n |
Deaths d |
Mortality d/n (95% Cl) |
|---|---|---|---|---|---|
| a. Cohen | 1974-1978 | PaO2< 50 torr, FiO2=1.0 | 81 | 47 | 0.58 (0.47,0.69) |
| b. Brett | 1977-1979 | PaO2<50 torr, FiO2=1.0 | 13 | 2 | 0.15 (0.03,0.46) |
| c. Bernbaum | 1976-1980 | PaO2<50 torr, FiO2=1.0 | 37 | 19 | 0.51 (0.35,0.68) |
| d. Dworetz | 1980-1981 | A-aDO2>600 torr | 5 | 5 | 1.00 (0.46,1.00) |
| e. Wung | 1983-1985 | A-aDO2>600 torr | 14 | 0 | 0.00 (0.00,0.27) |
| f. Ortega | 1984-1986 | Oxygenation Index > 40 | 26 | 16 | 0.61 (0.41,0.79) |
| g. John | 1983-1985 | A-aDO2>600 torr | 18 | 3 | 0.17 (0.04,0.42) |
| h. Nading | 1983-1985 | A-aDO2>600 torr | 5 | 0 | 0.00 (0.00,0.54) |
| i. Dworetz | 1986-1988 | A-aDO2>600 torr | 9 | 1 | 0.11 (0.00,0.49) |
| Group 1. Summary of all studies (a-i) | 208 | 93 | 0.45 (0.38,0.52) |
||
| Group 2. Studies conducted 1983 and after (e-i) | 72 | 20 | 0.28 (0.18,0.40) |
||
| Group 3. Studies of infants mananged without hyperventilation (e,i) | 23 | 1 | 0.04 (0.00,0.24) |
||
For surviving infants, we assign a multiattribute utility score, based on the infant's ultimate health outcome state (Figure 1 B) to represent quality of life. Torrance and his colleagues developed this score for use in cost-effectiveness evaluation of neonatal intensive care for low-birth-weight infants.16,17 It represents a child's functional capability and quality of life in terms of four factors: physical mobility and activity; self care and role activity; emotional well-being; and social activity; and health problems. The investigators standardized the scale through interviews with a random sample of parents of Hamilton, Ontario schoolchildren. Parents stated their preferences for various health outcome states relative to other health states and to the reference states, "healthy" and "dead," by selecting a position for each state on a linear scale corresponding to their feeling about the desirability of that state. Note that parents rated severe disability (utility -0.39) to be worse than death (utility 0.0). Table 1 lists the attributes and utility values assigned to each health outcome state in the decision tree.
Probabilities of Chance Events
We reviewed the medical literature to obtain estimates of the probabilities for chance events modeled in the decision tree (Table 2 and Table 3). Because the two randomized trials comparing ECMO and CMT include a small number of patients3,4 and long-term follow-up has not been reported for these patients, we used data from uncontrolled studies of patients treated with either ECMO or CMT.
To insure comparability of patients, we attempted to include only studies in which infants might be considered eligible for ECMO. We considered studies limited to term or postmature infants (gestational age >35 weeks, birthweight > 2 kilograms) with idiopathic or secondary PPHN. One study of ECMO therapy reported outcomes for some premature infants and children >1 year of age,18 but we used only the data for the term neonates. We did not include studies which reported solely on the use of ECMO for managing respiratory failure in newborns with congenital diaphragmatic hernias.19, 20, 21, 22, 23
Several criteria were used to select studies so that the severity of illness was comparable for infants treated with each type of therapy. Many early studies of ECMO used moribund clinical status or acute clinical deterioration as the primary indication for treatment.1,18,24 More recent studies used physiologic indices of cardiorespiratory function, such as elevation of the alveolar-arterial oxygen gradient (A-aDO2)25,26 and elevation of the oxygenation index (Ol). 27
To select studies of CMT (Table 2), we used the following criteria: elevation of A-aDO2, ³ 600 torr;5,7,8,28 elevated OI > 40;29 or reduced arterial partial pressure of oxygen (PaO2) to less then .50 torr despite high inspired oxygen content (FiO2 = 1.0) and maximal ventilatory support.30,31,32,33 We choose the last criteria based on a report demonstrating its equivalence to the other physiologic indices in identifying neonates at high risk for death with CMT.34 We excluded data on several infants in two studies of CMT because they did not meet the selection criteria of elevated A-aDo2 > 600 torr.8,28 We excluded other studies of infants treated with CMT, because we could not verify that these infants met our selection criteria.35,36,37,38,39
We calculated the probabilities for various events with each treatment by combining data on patients from all studies that met our inclusion criteria. The contribution of each study to a probability estimate was proportional to its sample size. We used a binomial approximation40 to estimate the 95% Cl interval (95% Cl) for each estimated probability. For some events, the literature reports only the combined probability for two events occurring together. For example, no distinction is made between death from PPHN and death from CVA. To estimate separate probabilities for each event, we used conditional probability algebra.41 We were not able to adjust for varying lengths of follow-up in different studies or missing data for patients who were not available for long-term follow-up, which averaged 14 percent in studies of CMT and 18 percent in ECMO studies. We assumed that outcomes for infants who were not available at the time of follow-up were similar to the outcomes for infants with whom the investigator maintained contact.
Risk of cerebrovascular accidents: Among 715 neonates in the National ECMO Registry,1 intracranial hemorrhage, recognized by cranial sonography, occurred in 14% (95% CI: 10%, 16%). Intracranial hemorrhage has not been reported in full-term infants in studies of CMT,5,7,28,29,30,31,32,33 perhaps because, prior to the use of ECMO, physicians did not routinely screen full-term neonates with PPHN for intracranial hemorrhage.42 We estimated the incidence of intracranial hemorrhage in infants treated with conventional therapy using data from a study of infants treated with ECMO in which the investigators routinely performed both cranial computed tomography and cranial sonography before and during ECMO.10 Intraparenchymal hemorrhages were found in 2 of 201 infants prior to starting ECMO (1%, 95% Cl: 0%, 4%).
In several studies of infants treated with ECMO, 10 of 81 survivors had non-hemorrhagic cerebral infarctions detected by computed tomography (12%; 95% Cl: 6%, 22%).9,18,24 Among infants treated with CMT, cerebral infarction, like intracranial hemorrhage, has not been frequently reported. In one study of conventional therapy, investigators routinely evaluated neonates with computed tomography43 and found 1 neonate with a biparietal occipital infarction among 14 surviving infants (7%, 95% Cl: 0%, 36%).
Table 3: Summary of Baseline and Threshold Probabilities for chance Events Used In Comparing Extracorporeal Membrane Oxygenation (ECMO) and conventional Medical Therapy (CMT)
The threshold probability is the probability at which the expected utility of the two strategies is equal, given baseline values for all other vanables. The probabilities of death due to PPHN with ECMO and CMT are adjusted to account for deaths due to cerebrovascular accidents (see Methods). 95% Cl: 95% Confidence Interval. NF indicates that a threshold probability for this event was not found. In these cases, EOMO is the preferred treatment for all values of the variable.
| Probability (95% Cl) |
Threshold probability |
|
|---|---|---|
| Intracranial Hemorrhage - ECMO | 0.14 (0.10,0.16) | 0.71 |
| Intracranial Hemorrhage - CMT | 0.01 (0.00,0.04) | NF |
| Cerebral Infarction - ECMO | 0.12 (0.06,0.22) | 0.47 |
| Cerebral Infarction - CMT | 0.07 (0.00,0.36) | NF |
| Death Due to PPHN - ECMO | 0.08 (0.06,0.11) | 0.42 |
| Death Due to PPHN - CMT | 0.17 | |
| Death Due to PPHN - Group 1: Summary of all studies | 0.43 (0.36, 0.50) | |
| Death Due to PPHN - Group 2: Studies conducted 1983 and after | 0.27 (0.1 7,0.39) | |
| Death Due to PPHN - Group 3: Studies of Infants managed without hyperventilation | 0.04 (0.00,0.24) | |
| Death due to Cerebrovascular Accidents | 0.48 (0.42,0.63) | NF |
| Profound Disability Due to Intracerebral Hemorrhage | 0.21 (0.06,0.51) | NF |
| Profound Disability Due to Cerebral Infarction | 0.58 (0.26,0.84) | NF |
| Profound Disability Due to PPHN | 0.02 (0.00,0.07) | NF |
| Developmental Disability - ECMO | 0.14 (0.08,0.22) | 0.57 |
| Developmental Disability - CMT | 0.21 (0.14,0.32) | NF |
| Chronic Lung Disease - ECMO | 0.11 (0.05,0.21) | NF |
| Chronic Lung Disease - CMT | 0.18 (0.09,0.32) | NF |
Risk of death
The National ECMO registry has reported short-term outcomes for 715 neonates treated with ECMO from 1980 to 1987. The overall mortality in these infants was 19% (95% Cl: 16%, 22%). However, among 99 infants with intracranial hemorrhage, diagnosed by cranial sonography, 52 infants died. Some of these deaths were probably due to intracranial hemorrhage in infants who would have survived PPHN itself; also, some infants may have survived their intracranial hemorrhage but died from PPHN or its associated conditions. To account for this, we modeled death from CVA and PPHN as separate events and used conditional probability algebra41 to estimate the specific mortality associated with either PPHN or CVA. We assumed that infants who survive a CVA face the same risk of death from PPHN as other infants. Because there were no reports of mortality with cerebral infarction, we assumed its mortality to be the same as intracranial hemorrhage. Thus, we estimated 8% (95% Cl: 6%, 11%) mortality from PPHN in infants treated with ECMO and 48% (95% Cl: 42%, 63%) mortality from CVA's for infants who suffer these events.
Among 208 neonates in 8 studies of PPHN treated with CMT (Table 2) 5,7,8,28,29,30,31,32 overall mortality was 45 percent (Group 1). Several investigators have suggested that the outcomes of PPHN with CMT have improved in recent years,57 particularly in neonates treated without hyperventilation.5,8 Therefore, we considered two subgroups of infants among those meeting our inclusion criteria: infants managed with conventional therapy in studies conducted in 1983 and after (Group 2: 28 percent mortality); and infants managed without hyperventilation (Group 3: 4 percent mortality). Table 3 lists the mortality for each of these groups with corrections for the mortality attributable to CVA's, using the same model and assumptions discussed above for ECMO.
Risk of disability
Children who survive PPHN may develop profound disability because of CVA's or from complications of PPHN and its associated conditions.44 We calculated the probability of profound disability from the prevalence of children identified as profoundly disabled or who had Bayley development index scores £ 50.45 We also assumed that the chance of profound disability was the same in survivors of CVA's regardless of the type of treatment. Combining data from several studies reporting outcomes of infants treated with either or ECMO, 9,18,24 we estimated the risk of profound disability to be 21% (95% Cl: 6%, 51 %) in infants following intracranial hemorrhage, 58% (95% Cl: 26%, 84%) with cerebral infarction and 2% (95% Cl: 0%, 7%) for PPHN with CNS complications.
To estimate its probability, we defined neurodevelopmental disability to mean an infant with a Bayley score of 50--7046 or a case history suggesting moderate cerebral palsy or mental retardation.18 The average reported prevalence of such developmental disabilities was 14% (95% Cl: 8%, 22%) in infants treated with ECMO 18,24,46,47 and 21% (95% Cl: 14%, 32%) in infants treated with conventional therapy.30,31,32,33
Published descriptions of patients with chronic lung disease following treatment for PPHN ranged from failure to thrive with associated developmental delay47 to prolonged requirement for supplemental oxygen.42 We choose the latter as the minimum criterion for including patients when we estimated the probability of chronic lung disease as 11% (95% Cl: 5%, 21%) in infants treated with ECMO 18,24,46,47 and 18% (95% Cl: 9%, 32%) in conventionally-managed infants. 29,30,32
Disease severity is an important variable in our model. Because it is reasonable to expect that the probability of complications correlates with the severity of illness, we indexed the probability of complications to the probability of death for conventionally-managed PPHN. When the mortality of PPHN with conventional therapy was 43 percent, the summary mortality from all studies (Table 3), we set the probability of all complications to their respective baseline values (Table 3). Then, using a simple linear interpolation, we decreased the probability of a complication as the probability of death from PPHN decreases. We did not adjust the probability of cerebral infarction and intracranial hemorrhage in neonates treated with ECMO, because of the possibility that these are complications of the therapy itself. 44,48
Results
Using the baseline probabilities shown in Table 3 and the expected mortality for PPHN treated with conventional therapy of 43 percent (Group 1), the expected value of ECMO is 0.63 quality-adjusted survival vs. 0.41 quality-adjusted survival for CMT. Because cerebrovascular complications occur less frequently with CMT, most deaths for these infants result from complications of PPHN. In neonates treated with ECMO, the predicted mortality is 19 percent, of which 8 percent is a consequence of PPHN and 11 percent is a consequence of cerebrovascular complications. Profound disability is expected in 6 percent of infants treated with ECMO compared to 2 percent of conventionally-managed neonates. The proportion of patients with either developmental disability or chronic lung disease is similar for ECMO and CMT. However, the proportion of infants with good outcomes is better with ECMO than CMT (55 percent vs. 32 percent).
Figure 2. One-way sensitivity analysis comparing ECMO and conventional medical therapy (CMT). On the y-axis, quality-adjusted survival varies for each therapy as the expected mortality from PPHN in infants managed with conventional therapy varies along the x-axis. The numbered points on each curve correspond to the groups of studies of conventional therapy presented in Tables 2 and 3. The horizontal bars show the estimated 95% confidence interval for each group. At the threshold mortality of 17% (dashed line), the expected utility of ECMO and CMT are equal. Above this threshold, ECMO is the preferred treatment (Groups 2 and 3). Below this threshold, CMT is the preferred treatment (Group 3).
The decision to perform ECMO depends on the severity of PPHN as measured by the probability of death from PPHN for conventionally-managed infants. Figure 2 shows a one-way sensitivity analysis in which quality-adjusted survival for each treatment changes as the probability of death from PPHN for conventionally managed infants is varied from 0 to 100 percent. All other variables either remained constant at their baseline values or changed as the probability of death from PPHN changed (see Methods). When the probability of death from PPHN is 17 percent (dashed line), the expected utility of both therapies is equal. ECMO is the preferred therapy if the predicted mortality from PPHN is above the threshold and CMT is preferred if mortality is below the threshold.
Figure 2 also shows the expected outcome for the three groups of studies described in Tables 2 and 3. The numbered point depicts the average mortality for each group and the bar is the 95 percent CI interval for the estimate. Group 1 summarizes all included studies of CMT. Within the range of the estimated 95 percent Cl interval for these infants, ECMO would be preferred to CMT. Group 2 infants are from studies conducted in 1983 and after. If the mortality of 27 percent for infants in Group 2 accurately reflects the performance of CMT at the present time, then ECMO is still the preferred treatment for these infants. However, the lower 95 percent Cl interval coincides with the threshold value of 17 percent mortality with conventional treatment. The advantage of treating an infant with ECMO, as measured by the difference in quality-adjusted survival between the two treatments, diminishes as mortality approaches this threshold. For infants in Group 3, those from centers using nonhyperventilation techniques, conventional therapy is preferred based on the reported mortality with medical therapy of 4 percent. The 95 percent Cl interval for Group 3 crosses the threshold line, but this may he due to the small number of infants reported in this group. If similar outcomes were observed in another 25 infants, then CMT for PPHN would be clearly optimal in these centers.
Table 3 shows threshold probabilities for other events, calculated by assuming an expected mortality of 43 percent with conventional therapy (Group 1). Because ECMO is the preferred therapy for these infants, we find thresholds only when we raise the probability of events which make ECMO appear worse. For example, the baseline estimate of the probability of intracranial hemorrhage is 14 percent (upper 95 percent Cl limit, 16 percent). When this probability exceeds 71 percent, CMT becomes the preferred treatment. We do not find thresholds when we lower the probability for events which make CMT appear more efficacious than ECMO, such as the probability of death or profound disability from a CVA. However, when the probability of profound disability from PPHN is greater than 83 percent, conventional therapy is the preferred strategy; when the chance of profound disability is too high, poor quality of life among survivors negates the benefit of improved survival with ECMO.
Figure 3 shows how assuming the best and the worst about ECMO affects the decision to use it once an infant's probability of dying from PPHN is known. We performed this analysis to represent the impact of uncertainty in our estimates for the probability of intracranial hemorrhage and cerebral infarction with ECMO, the probability of profound disability with either intracranial hemorrhage or cerebral infarction, and the probability of developmental disability or chronic lung disease with ECMO. We set the probabilities for these complications at the lower limit of their 95 percent Cl intervals for the best-case scenario and at the upper limit of their 95 percent Cl intervals in the worst-case scenario. In the base-case scenario, we set these parameters at their baseline values (Table 3).
For each scenario, Figure 3 shows the threshold line for the efficacy of ECMO at which, for any particular value of expected mortality with conventional therapy, one should be different to the choice between ECMO and conventional therapy. The efficacy of ECMO is the proportion by which ECMO reduces the expected mortality of PPHN if man-aged with conventional therapy. For example, for Group 1 patients, the expected mortality is 43 percent with conventional management. If the expected mortality in neonates managed with ECMO is 8 percent, then the efficacy of ECMO is 81 percent.
The lines for the best-case and the worst-case scenario divide the space into 3 regions. For infants in Group 3, in the area to the left, CMT is clearly the optimal strategy. For infants in Group 1, in the area to the right, ECMO is the preferred therapy. For infants in Group 2, those in the middle area, ECMO also appears to be the preferred therapy. However, because data for the probability of certain complications of ECMO are imprecise, this choice is not as clear as for Group 1 and Group 3 newborns. As these important data become known with greater precision, the size of the middle area will decrease and so will the level of ambiguity in the decision for Group 2 patients.
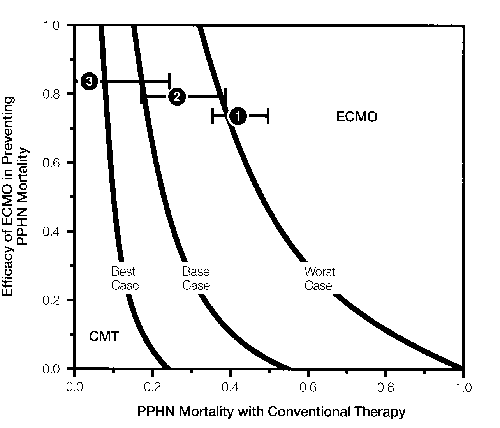
Figure 3. Three-way sensitivity analysis comparing ECMO and CMT. The x-axis is the expected mortality from PPHN in conventionally-managed infants. The y-axis is the efficacy of ECMO, which is the proportion by which ECMO reduces PPHN mortality from that expected in con ventionally-managed patients. Based on dafa for the risk of complications with ECMO, the three curves represent threshold lines for best-case, base-case and worst-case scenarios (see text). The numbers in each area correspond to the groups of studies of conventional therapy presented in Tables 2 and 3. In area to the right (Group 1), ECMO is the preferred therapy; CMT is the preferred therapy in the area to the left (Group 3). For Group 2 infants (middle area), the choice between ECMO and CMT is ambiguous because of imprecision in the data for ECMO complications.
Discussion
We have presented a decision analysis model that compares ECMO and CMT for treatment of PPHN. For infants in whom the expected mortality with CMT is high, the model predicts that ECMO will result in improved survival. This finding is consistent with the results of two previous randomized clinical trials comparing ECMO to CMT.3,4 However, as for other technologies used in critically-ill neonates,6 ECMO is a risky choice. The model predicts that more ECMO-treated children will die of the cerebrovascular complications of ECMO than of PPHN itself (12 percent vs. 7 percent), and more survivors will be profoundly disabled with ECMO (6 percent) than with conventional therapy (2 percent). Nevertheless, ECMO is beneficial for severely-ill infants because many of these infants, who might otherwise die with conventional therapy, will survive with good outcomes if treated with ECMO.
For less sick-infants, ECMO is not clearly favored over conventional therapy for PPHN. As Figure 3 shows, the choice of treatment is sensitive to the imprecision in our present data on the risk of complications with ECMO. For example, in Group 2 infants (27 percent expected mortality with conventional therapy), we cannot be as certain that the benefits of ECMO therapy definitely outweigh its risks. For Group 3 infants (4 percent expected mortality) from centers reporting success in managing PPHN with nonhyperventilation techniques, conventional therapy is preferred. Even if ECMO therapy prevents every death in such infants, the risk of disability from cerebrovascular complications with ECMO would outweigh the benefit of improved survival.
The probability that an infant will die of PPHN if treated with CMT is probably the most important factor to consider in deciding to use ECMO. A major research priority is the development of a clinical prediction rule49 that reliably estimates the probability of death for neonates with PPHN if treated with CMT. Several eligibility rules have been proposed to identify severely-ill infants with PPHN.4,25,27,34 However, these rules may not predict mortality accurately when applied at different centers5,7 and do not stratify the risk of death for less-sick infants. Also, because investigators used retrospective data to develop many of these eligibility rules, these data may not reflect outcomes presently obtainable with state-of-the-art ventilator management techniques.50
It is also important to study how CMT for PPHN can be improved. Two modalities that deserve particular attention are the use of non-hyperventilation techniques5,8 and high-frequency oscillatory ventilation.51 We recommend that these studies be randomized and include infants treated with conventional hyperventilation techniques as controls, so that differences in outcomes can be clearly attributed to different techniques of ventilator management. Because ECMO has proven efficacious for the sickest infants, many clinicians may feel ethically compelled to offer ECMO for these babies.52 Still, such studies can be conducted in centers where ECMO is readily available to infants who don't respond to conservative management.
Other issues deserve further study as well. The incidence of severe PPHN among infants who are born at facilities with high-level neonatal care centers is reportedly lower than among neonates born elsewhere.50 This may provide clues to important factors in the early management of infants at risk for respiratory distress. Also, it is possible that meconium aspiration syndrome, which is associated with PPHN, may be easily preventable with improvements in prenatal or perinatal management. The incidence of PPHN and meconium aspiration syndrome in the United Kingdom appears to be less prevalent than in the United States.53 Also, a recent study reports a significant decrease in the incidence of meconium aspiration syndrome and associated deaths in U.S. Army Medical Centers from 1973 through 1987, even though the incidence of meconium-stained amniotic fluid did not change during this time.54 The reduced incidence of meconium aspiration syndrome may be due to the use of intrapartum oropharyngeal suctioning and postpartum intratracheal suctioning of meconium-stained newborn infants.53,54
A primary limitation of the present analysis was the lack of precise data for some important events in the model. For example, although intracranial hemorrhage is an important source of morbidity and mortality with ECMO, reported series do not distinguish between neonates who die of intracranial hemorrhage and neonates with intracranial hemorrhage who die from PPHN or its associated etiologies. In the studies we reviewed, many survivors were unavailable for later follow-up of outcomes. We assumed their outcomes to be the same as children who were available, which may have resulted in systematic overestimation or underestimation of morbidity for either therapy. Also, most series reporting on either ECMO or conventional therapy report follow-up only for the first few years of life. Neurodevelopmental disabilities identified in early childhood, unless profound, may poorly predict eventual functional status55 and conversely children who appear to be normal in early screening may eventually develop functionally-important disabilities.56 Thus, our model may underestimate the impact of neurodevelopmental disabilities for both ECMO-treated and conventionally-managed children. Figure 3 illustrates the impact of imprecise data for complications of ECMO on our model.
While our model considers differences in quality of life among survivors, we do not account for possible differences in their life expectancy. If we multiply the life expectancy of survivors by quality-adjusted survival, we would obtain the expected value of each strategy in quality-adjusted life years.11 This would not affect the results of our model if the average life expectancies of all survivors were equal. However, our model would not be strictly accurate if the life expectancy of disabled individuals were different from that of healthy individuals. For example, if the life expectancy of a profoundly disabled child were shorter than for a healthy child, the expected value of each therapy would be reduced in proportion to the expected number of profoundly disabled survivors.
We chose not to represent outcomes in terms of quality-adjusted life expectancy because we did not have accurate data on the life expectancy of individuals with various types of disabilities. We were also concerned that the life expectancy of healthy ECMO survivors, who usually have their right common carotid artery permanently ligated, might be different from that of healthy survivors of conventional therapy with intact vessels. When we performed sensitivity analyses on our model, making various assumptions about life expectancy, we found that this didn't alter the interpretation of the model. In general the magnitude of discrepancies arising from ignoring possible differences in life expectancy were less important than uncertainties due to imprecision in the data on the risk of complications.
We have reviewed the medical literature and built a decision-analysis model which examines available information on the risks and benefits of ECMO and conventional therapy in the management of infants with PPHN. ECMO appears to offer significant advantages, in terms of improved quality-adjusted survival for infants who are at high risk of dying with conventional therapy. However, we need better clinical prediction rules that can accurately represent differences in severity of illness for infants with PPHN and a better understanding of the impact of improvements in preventive and conventional therapy for PPHN. Meanwhile, ECMO should be used cautiously in less-sick infants.
Acknowledgments
This work was supported in part by the Agency for Health Care Policy and Research.
References
- Toomasian JM, Snedecor SM, Oornell HO et al.: Natonal exper once wth extracorporea membrane oxygenation for newborn respiratory failure. Oata from 715 cases. ASAc Trans 34:140-7. 1988.
- Hess KF, Bartlett RH: Extracorporeal membrane oxygen-at on: An exper mental protoco becomes a clinical service. Adv Pediatr 36:117-35, 1989.
- Bart ott RH, Roloff Ow, cornell RO et ak: Extracorporeal circulation in neonata respiratory fa. ure: A prospective randomized study. Pediatrics 76:479-87, 1985.
- ORourke PR, crone RK, vacanti JP et al.: Extracorporee membrane oxygenation and conventional medico therapy in neonates witn persistent pulmonary hypertens on of the newoorn: A prospective randomized study. Pediatrics 84:957-963, 1989.
- Oworetz AR, Moya FR, 5abo B et al.: Survival of ofants w th persistent pulmonary hypertension without extracorporeal membrane oxygenation. Pediatrics 84:1 -8, 1989.
- Nelson NM: On hummingo rds, extracorporeal membrane oxygenat on, and IBM. Peoiatrics 85:374-376, 1990.
- Nod ng JH: Histor ca controls for extrocorporeal memorane oxygenation in neonates. crt Osre Med 17:423-0. 1989.
- Wung j-T, James LS. Kilcnevsky E et 01.: Management of infants w th severe respiratory to ~re and persistence of fetal c rculation, witnout hyporvent at on. Pediatrics 76:488- 494, 1985.
- Schumacner HE, Barks .~0, Jonnaton Mv of 01.: R gnt-siood orain esions in nfants fo ow 09 extracorporeal membrane oxygenation. Pod atrcs 82:155-61, 1988.
- Taylor GA, F tz OH. Glass Pet Ok: ci of cerebrovascular iniury after neonata extrocorporeo membrane oxygenat on: imp cotions for ne.~rooeveIopmontal oAcome. Am J Roentgonol 153:121-6, 1989.
- Sox HO, Blatt MA, H gg ns M~ ot a.: Menical Becision Making. Boston, Bufterworths, 1988.
- Pa..Ker SO, Kass rer JP: Becision analysis. N Engi J Moo
316:250-8, 1987. - Fox WW. 0~ara 5: Persistent pulmonary nyportension in the neonate: 0 agnes sand management. Pediatrics 103:505-13, 1983.
- Duara S. Oewitz Mr-f. Fox WW: Ose of mecnanical ventilation for c n ca management of porssfont pu[monary hypertension of too newborn. Oin cs n Per nafology 11:641-652,1984.
- White JJ. Anorows HO, H semberg E. eta.: Prolonged reap rafory support in newborn nfants wth a membrane oxygenator. Surgery 70:288-94, 1971.
- Torrance OW, Boy o MH, Horwooc SP: Application of mu tiottr o~.te .~fiiity theory to measure soc 01 preferences for heath states. Operations Resoaron: 30-1043-1069, 1982.
- Boyle MB, Torrance OW. Sinclair JO ot a.: Economic evaluation of neonoto otensive care of very-low-birtn-we.ght infants. N Eng ,. Med 308:1330-7, 1983.
- Towno RH, Loft 1T, HicKs GA of 01.: Long-term fo ow-~.p of infants 000 chiloren treated witn oxtracorporoa. memorane oxygonaf on (ECMO): A prolim nary report. J Pediatr Surg 20:410-4, 1985.
- Weber TB. Ounnors RH, Penninglon 00 of a.: Neonatal o aphrogmat c horn a. An mprov ng outlook wifn oxtracorporeal momorano oxygenation. Arcn Surg 122:615-8, 1987.
- Stolar OJ, B on PW, Stalcjp SA: Ext racorporeal membrane oxygonafon and congenital d aporagmatic 00mb: Mooif cation of foe pumonory vasoactve profile. J Pod atr S~rg 20:681 -3, 1985.
- Joonsfon PW, Basoner B, Lioerman B of 01.: GImbal use of ext racorporea membrane oxygenation n toe treatment of persistent pulmonary hypertenson following surgical repair of congenital o aphrogmat c horn a. J P00 atr Surg 23:908-12. 1988.
- 80155 K, Manning P. 0 dham KT et a.: Reversal of mortality for congen to dioporagmotic horn a w th BOMO. Ann Surg 209:225-30, 1989.
- Loogham MHJ. Krummel TM, Bart oft RH of 01.: Mortality w,th ext racorporeo membrane oxygenaton fo ow 09 ropa.r of congenital o,aphragmat c horn a in 93 infants. J Pod atr S~.rg 22:1150-4, 1987.
- Krummol TM, Greenfie,o U, K rkpafr Ok ev eta .: The ear y evaluation of survivors after oxtracorporea memorano oxygonat on for neonatal pu monary faiiure. j Pedratr S~rg 19:585-90, 1984.
- BOOK H, Anderson KB, Pearson GD of ai.: Orteria for oxtracorporeal momorane oxygonat on ,n a population of ofants w th persistent po monary nyperfension of foe newborn. J Pediatr Surg 21:297-302,1986.
- Short BL, Miller MY, Anoerson KB: Extracorporeal membrane oxygenation in foe management of respiratory failure in toe newborn. 0 n Per natal 14:737-48, 1987.
- 2Ort z RM, Gilley RE, Bart oft RH: Extracorporoal membrane oxygenation in pediatric resp rofory failure. Poo,atr 0 n Norto Am 34:39-46, 1987.
- ,~onn E. Roberts v, Burnard 60: Persistent pulmonary nypertension of tno newborn treotoo wito nyporventilat on: 0 n cal features and ro.,t come. Aust. Poooiatr. J 24:357-361. 1988.
- Ortega M, Ramos AD, PlotzKer AO eta.: Ear y prediction of ult mate outcome n newborn ofonts w fh severe resp ratory to jre.JPooiofrll3:744-7 1988.
- Oonen RS, Stevenson BK, Mo acnows~i N Ot 01.: Late moroidity among surv vors of respratory to ~re troatec wtn to az no. J Podiatr 97:644-647, 1980.
- Brett 0, 00Kb M, Leonard OH of 01.: Development to ow-L.p of hyperventilated neonates: Preliminary onsorvotions. P00 atr os 68:588-591, 1981.
- Borobaum JO. R~sse P, Sherican PH of a.: Long-term fo. ow-up of newborns w th persistent pulmonary nypertenson. Ort Oaro Moo 12:579-583. 1984.
- Se EJ. Gaines JA, 0 ~.ckmon 0 et a.: Pors stent fetal circulation: Neurodevo opmentol oAcome. AJOG 139:25-28. 1985.
- Marso TB, W Kerson SA, Goort LN: Extrocorporeal momorane oxygenation selection criteria: Porfio pressure of arferal oxygen vers~.s aveolor-arteria oxygen grac ont. Pooatrcs82:162-6, 1908.
- Ferraro B, Johnson BE, Ohong P of o.: Efficacy ano neurorogic outcome of profo~nd hypocapne c a Kabos s for the treatment of persistent pulmonary hypertension in infancy. J Podiatr 105:457-461, 1984.
- ...evin Dl. Heymono MA, K tferman JA of 01.: Persistent pulmonary nypertension of tne newborn ofont. J Pediatr 89:626-630, 1976.
- Hageman JR. Aoams MA, Gardner TB: Pulmonary comp cattions of hypervent, ation toerapy for pers,stent pulmonary hypertension. Grit Gore Med 13:1013-1014. 1985.
- Robertson NRC, Hallidio-Smth KA, Ba~s JA: Severe respiratory o stress syndrome m m oking cyanotic noartdisease inform babes. Lancet 2:1108-1110, 1967.
- Sass B, Goldberg SJ, Emmano~. des GO of a.: Pers stent pL monary vasc~. 00 00sf ruction in newborn ofants. Podiatr 78:610-615, 1971.
- Flciss J.j Stat stical Methods for Rates and Proportions, 2nd edition. New York, Jonn Wiley & Sons, 1981.
- Broke AW: Funoamenfo s of Applied Prooability Theory. New York, McGraw-H .1967.
- Gass P, Miller M, Short B: Morn a ty for survivors of ext racorporea membrane oxygenat on: Ne~rooovebopmenta Outcome at 1 year of ago. Pediatrics 83:72-8, 1989.
- Kbesh KW, Murpny TE, Sober MS eta.: GoreOral infarct on in pors stont p~. monary Oyporfonsbon of the nowoorn. AJOG 141:852-857, 1987.
- 0 ey RE, zw schenoorgor JB, Anorows AF of 01.: bntracranba hemorroago during oxfrocorpomai momorane oxygenation n neonates. Pod atr 03 78:699-704, 1986.
- Boyloy N: Manual for foe Boy ey Scoos of Infant Dove opmont. Now York, Too Psycoological Gorporat on, 1969.
- Toy or GA, F fz OR, Kapur S Ot 01.: Gerobrovaso.. or aco dents in neonates treated w th ext rocorporeal membrane oxygenation: Sonographic-patnologic corroiation. Am Roentgeno 153:355-61, 1989.
- Androws AF. N xon GA, Gluey HE of a.: One- to three-year outcome for 14 noonafo surv vors of extracorporoa membrane oxygenaf on. Peobotrics 78:692-8. 1986.
- Taylor GA, F fz OR, Miller MK of 01.: Intracran 01 oonormalities n infant treated w fh ext racorporea. momorane oxygenation: Imaging wifo 85 and OT. Radioiogy 165:675-8. 1987.
- Wasson SB, Sox HO, Neff RK of 01.: GImbal proc of on rules: Applications ons and met nooological stanoords. N EngI.. Mod
313:793-9, 1985. - Gross I: The oxtracorporoal momorano oxygenat on cobato. Pen afros 85:383-4, 1990.
- Garter JM, Goratmano BR, GlarK RH at a.: H.gh-freguoncy nan I atory vontiiation ann oxtracorporoal membrane oxygenation for too treatment of ac..fo neonatal respiratory failure. Pediatrics 85:159-164, 1990.
- Snort Os Too ext racorporoal membrane oxygenation decato. Pediatrics 85:300-381, 1990.
- Persistent feta. c roulation ann oxtracorporeal membrane oxygenation (ecitor al). Lancot 2:1289-91, 1988.
- W awe TE, Toggle JM, Turner 05: Macon ~m aspiration syndrome: Have we made a o fferonoo? Peobatrics 85:715-721. 1990.
- Holdon RH: Proc of on of mental rofardaf on in ofancy. Mental Rotardat on 10:26-30, 1972.
- Bergman I, Banor RE, Barmana MA et al.: Intracerebral hemorrhage in the full-term infant. Pediatrics 75:488-496, 1985.
Extracorporeal Membrane Oxygenation:
Cost, Organization, and Policy Considerations
Rachel M. Schwartz, M.P.H.
Katharine K. Wilirich, B.A.
David E. Gagnon, M.P.H.
National Perinatal Information Center
Abstract
The cost of ECMO has not been studied extensively from the perspective of individual per case cost or from the perspective of system costs. In this research, the authors explore cost from both perspectives and, for the latter, place it within the policy context of the tremendous expansion which has occurred and is planned to occur.
In order to estimate acute cost per case for ECMO and identify whether it is more or less expensive than conventional care, some assumptions must be made about candidates for ECMO and the comparison groups. The authors use one primary assumption throughout: neonatal EMCO candidates have an 80 percent mortality rate without ECMO and therefore most would die with conventional care. The key finding for individual cost per case is that, in the acute phase of care, conventional care patients who either live or die are less costly than ECMO cases. In addition, to be able to draw conclusions about costs per survivor or cost-benefit of ECMO, better documentation concerning the immediate, moderate, and long-term mortality and morbidity is needed.
Estimates of annual system-wide costs vary dramatically depending on the method used. The system cost for ECMO based on per case costs is at least $90 million per year for cases that occur no more frequently than 1 in 1309 births (if all ECMO treated cases survive the acute phase of care). If we subtract the potential conventional care cost of this group (assuming 80 percent die), the excess is about $50 million dollars.
System-wide costs would rise dramatically if the reported expansion plans occur. Using organization data on the number of cases who could be seen in 15 ECMO units surveyed for this study, the authors conclude that further expansion cannot be supported by demand estimates and would therefore create unnecessary costs to the system.
As the application of high technology in the care of newborns becomes increasingly prevalent, and as health care costs assume a greater proportion of the United States gross national product, policymakers and insurers are prevailing on providers to document the costs and benefits of care.
The use of ECMO in newborns, a relatively new surgical technology for the treatment of severe acute lung disease, has grown geometrically in the last 5 years. The ECMO Registry recently documented that 1,006 cases had been treated in 1989 for a total of 3,5971 in the last 10 years. The number of centers in the United States providing this service has risen from 3 in 1982 to 57 in 1989.2 Most recently, a 1988-1989 survey of obstetrical hospitals (on which these authors collaborated with the Maternal and Child Section of the American Hospital Association) revealed that of the 668 hospitals with neonatal intensive care units who responded to the survey, 41 had ECMO units (children's hospitals were not included). Of the 627 respondent centers who did not offer ECMO, 31 said they planned to open an ECMO unit within the next 18 months. Such an increase would expand the number of facilities by more than 50 percent within the next 2-3 years. These increments raise issues about the cost of care, the presence of sufficient cases to support this expansion and permit clinicians to provide quality care, and finally, the possible inefficiencies which result from the expansion of a service infrastructure. To develop meaningful health policy in this area, some answers are needed.
In this paper, we examine several aspects of the cost of ECMO. These include: the cost of operating an ECMO unit, the marginal costs of ECMO compared to neonatal intensive care unit costs, and the acute inpatient cost per case for ECMO patients compared to that of patients meeting ECMO criteria who do not receive ECMO. While lifetime cost of newborn ECMO patients and conventional care patients is not a part of the analysis, we explore the factors necessary to create estimates. Finally, the system-wide health cost are explored with regard to the expansion and existing capacity noted above.
Materials and Methods
Operating and Marginal Costs
To determine the cost of operating an ECMO unit, we identified the centers in the United States that had operated ECMO units for 5 years or more. A list of hospitals was provided by the ECMO Registry. Of the 19 centers on the list, 18 were in current operation and all these were contacted by telephone to complete a four-page survey. Fifteen hospitals responded, a response rate of 83 percent.
The survey requested information on utilization, start-up, and current operations including budget, staffing, equipment purchase and maintenance, and billing policies.
Because many hospitals were unable to provide separate ECMO budgets, the calculation of cost of operating an ECMO unit was done in three separate ways. In our analysis we examined actual budget costs; we calculated cost based upon the reported components (personnel, equipment, maintenance and disposables); and finally, we used reported charges and length of stay to calculate the marginal cost of ECMC cases. These three approaches provide a range of cost per case for the ECMO component of care and total cost per unit.
Estimates of marginal costs were only available from a subset of hospitals (five) who operate ECMO units and who are members of the National Perinatal Information Center (NPlC). These figures came from audited Medicare Cost Reports. For each hospital, the cost of the ECMO unit was divided by the total NICU operating cost to identify the percentage associated with ECMO.
Cost of ECMO Patients (Acute Phase)
The cost of treatment for ECMO patients was derived from four sources. We performed a special analysis of NPIC hospitals who offer ECMO, two unpublished sources (with special permission, )Ochsner4 and University of Michigan5), and one published source, Pearson and Short.6
To identify cases at seven NPIC member hospitals (in 1988), the ECMO procedure code from the ICD-9-CM7 code book was used to search the discharge abstract files for cases. NPIC member hospitals provide discharge abstract and billing information on 100 percent of all neonatal and obstetrical discharges every year they are members. Based on survey responses, we expected to identify over 50 cases; however, due to hospitals' failure to code cases using the ECMO procedure code, only 12 were identified and are presented here. The pooled analysis from the four sources includes a total of 89 infants who lived and 14 who died. Because the University of Michigan reported only cases of survivors, for some analyses these cases were dropped, leaving a total of 83 cases (lived and died).
Because information on the ECMO cases who die was only available on 14 cases across all sources, we did not perform a cost per survivor analysis which would combine the cost of those who died with those who lived and then divide by those who live. In addition, no attempt was made to add the differential cost of physician care when physician charges were not already included in the data. Thus the direction of the error is to make the ECMO cases appear less costly than they are.
Cost of Comparison Patients
To understand adequately the differential cost of ECMO treatment, it is necessary to ask how much it would cost to care for these patients using conventional approaches. Ideally, a cohort of cases would be assigned prospectively to treatment and control groups and not only the clinical information on mortality and morbidity, but also resource use would be presented. Due to ethical issues (discussed elsewhere8), there have been few studies of ECMO that are randomized prospective analyses and the two that have been published do not include information on cost and length of stay. However, Pearson and Short9 and Roloff10 (unpublished) have worked in this area, and their data are presented here. Finally, James11 provided statistics on 29 cases that met ECMO criteria at Columbia-Presbyterian Hospital.
We, however, did not wish to rely solely on clinical sources, especially when just one (Roloff) was part of a randomized prospective study (providing five non-ECMO patients). To supplement the current work in the field, we performed original analyses to identify both cases who lived and died who would meet the criteria. Analysis of ECMO-eligible newborns who would have died centered on the use of 1984 National Birth-Death File12 (a year when there were only 83 ECMO cases) to identify both how many ECMO eligible births by diagnostic category were likely to be born in a given year and the average length of life of those who die.
The 1984 birth-death file, which became available for public use in September 1989, is prepared by the National Center for Health Statistics (NCHS). The data file provides most of the information for all births and infant deaths in the United States up to 1 year of age, from the standard U.S. birth certificate, including data on birthweight, race, sex, and the information from the death certificate including the data necessary for this analysis—primary cause of death and up to 20 comorbid conditions contributing to the cause of death.
The analysis performed identified all infants who weighed over 2,000 grams and died of these diagnoses: persistent pulmonary hypertension, congenital diaphragmatic hernia, massive aspiration syndrome, severe respiratory distress syndrome, sepsis, total respiration failure, pneumothorax or barotrauma, and bronchopulmonary dysplasia. The diagnoses were identified through the use of ICD-9-CM diagnostic coding scheme which is used on the death certificates to code cause of mortality and comorbidities. The NCHS files include both an original coded cause of death and a recoded cause of death. The recoded cause of death is based on an automated algorithm designed to identify and consistently code the underlying cause of death when multiple causes are provided. Our analysis used the latter.
Because there were only 83 ECMO cases treated in 1984,13 it represents, in terms of overall statistics, a year prior to significant effects of ECMO. We therefore assumed that when looking at 1984 NCHS data, using current standards, we would find roughly 80 percent of the ECMO cases among those who died. The average length of life (ALOL), the time each infant was alive until it died, could then be used as a control or conventional-care group for the ECMO-eligible cases who actually die and for the died component of all cases together (lived and died). Finally, the number of cases identified divided by 0.8 (those expected to die on conventional treatment) provides an outside demand estimate. Using this approach we identified 2,241 ECMOeligible cases in the 1984 birth-death cohort file. Assuming this represents 80 percent of all ECMO cases, then 2,241 ¸ .8 or 2,801 would be the expected number of cases in the United States in 1984. Births were 3,669,288 in 1984. Thus for every 1,309 births there would be one ECMO case.
In order to insure that the national cohort looked as similar to the actual ECMO cases as possible, the distribution of cases by diagnoses from 715 cases14 in the ECMO registry was used to adjust the ALOL results. We found the adjusted data resulted in a shortened ALOL for cases treated by conventional treatment, and in the interest of a conservative analysis we used the unadjusted data in the analysis.
The birth-death file does not include cost of care data. We determined that the best sources of total charges for newborn care, described in terms of both birthweight and diagnoses, is the tertiary hospital component of a national data base designed and collected by these authors for a separate study.15 This 1985 data set, described elsewhere,16 included a stratified 28-hospital random sample of urban tertiary perinatal centers with over 900 births, drawn from the universe of 360 non-tertiary perinatal centers. This data set includes discharge abstract data, including birthweight, diagnostic codes and billing information on all infants who were either inborn or outborn in 1985 at these hospitals.
The 87,915 infant data set was used to identify the total hospital charges for ECMOeligible cases who were born or transferred to these hospitals and who either lived or died. We estimated that if ECMO cases were present in these hospitals in the ratios similar to those identified in the birth cohort file (1 for every 1,309 births), there would be at least 67 in total, 54 of whom would have died and 11 of whom would have lived (using the ratio of 20 percent live and 80 percent die). However, we expected to find more cases because tertiary hospitals receive maternal referrals and newborn transports of high-risk cases.
Table 1: Selected Survey Results n=15 (83% response rate)
*FTE=full-time equivalent.
**NICU=newborn intensive care unit.
***PICU=pediatric intensive care unit.
| Mean | Number of respondants | |
|---|---|---|
| Average Years in Operation | 6.2 | 15 |
| Average Number of Patients Per Year | 39.7 | 15 |
| Average Number of Patients Who Can be Cared for at Once | 2.9 | 15 |
| Average Length of Stay on ECMO | 6.0 | 15 |
| Average FTE* Staffing for ECMO | 4.13 | 15 |
| Percent Units Located in NICU** | 60 | 15 |
| Percent Units Located in PICU*** | 27 | 15 |
| Mean ECMO Budget | $349,167 | 6 |
Table 2: Calculated Cost Based Upon Components
*4.13 is the sum of FTE nurses, respiratory therapists, coordinators (usually nurses).
**$46,550 derived from $35,000 (average salary of all personnel types) x 1.33 (standard benefit package)1.33 is a standard benefit package.
| Mean | Factor | Total cost per year | |
|---|---|---|---|
| ECMO Personnel (n=15) | 4.13 FTE* | $46,550** | $192,251 |
| Equipment (n=9) | $41,288 | 5 Yrs. | $8,257 |
| Maintenance Per Year (n=9) | $3,272 | N/A | $3,272 |
| Disposables | $993 | 40 patients/yr. | $39,720 |
| Total | $243,500 |
Table 3: ECMO Cost Based Upon Survey-Reported Charges to the Patient
| Mean | Factor | Cost based on charges | |
|---|---|---|---|
| Average NICU Per Diem (n=7) | $1,040 | 6.0 days | $6,240 |
| Average ECMO Per Diem (n=13) | $1,480 | 5.0 days | $7,400 |
| Average Set-up Cost/Case (n=13) | $1,655 | 1.0 days | $1,655 |
| Average Disposables Per Case (n=10) | $933 | 1 per case | $933 |
| Total | $16,228 per case |
The primary analysis and the published and unpublished figures are presented from the perspective of all cases, survivors, and cases who died. As will be discussed below, every analytical decision was made to err in the direction of raising the cost of the conventional care group, thereby resulting in conservative findings.
Results
Survey Overview
Table 1 summarizes the 15 hospitals who responded to the telephone survey. Eight key descriptive variables are presented. On average, ECMO units at this group of hospitals were in operation for just over 6 years, saw roughly 40 patients per year and could care for 3 patients simultaneously. They reported keeping patients on ECMO for an average of 6 days. The average staffing was 4.13 fulltime equivalents (FTEs) per unit. This figure includes registered nurses, respiratory therapists, and ECMO coordinators. Although we have not presented data on how many of the staff were dedicated to the unit or drawn from the NICU or PICU, it appears that the staffing model was quite mixed, with some facilities relying on NICU staff and others hiring dedicated staff. The figure presented is a composite of both.
Most ECMO units are physically located in the NICU (60 percent) with fewer (27 percent) located in the PICU. A small percentage (13 percent) were within neither. Budgets are largely embedded within the NICU or PICU budget, and only 6 of the 15 respondent hospitals were able to provide a dollar budget. The amount averaged $349,167 for the 6 reporting hospitals.
Operational and Marginal Costs of Running an ECMO Unit
In addition to collecting data on a total budget, we asked for information about equipment costs, maintenance, and disposables. In general, a larger number of hospitals were able to respond to these questions. These responses were used to calculate the cost of an average unit based upon its components and provide another gauge to determine the probable operating costs in a unit staffed by 4.13 FTE professionals with an average of 40 cases per year. The calculated cost based upon the components on average would be $243,500 per year (see Table 2).
Table 4: Comparison of Operational Costs Using Three Approaches
*From Table 2.
**Average number of cases in six hospitals responding to ECMO budget questions.
| Actual budgeted ECMO costs n=6 |
Calcualted cost based upon components* n=9-15 |
Calcualted cost based upon charges only n=7-13 |
|
|---|---|---|---|
| Annual Cases/Hospital | 47.5** | 40 | 40 |
| Average Cost Per Case | $7,350 | $6,087 | $16,288 |
| Total | $349,167 | $243,500 | $651,520 |
Table 5: Marginal Cost of Operation Compared to NICUin selected Hospitals
*Medicare Cost Report, 1987.
**Reported budget from survey.
***Estimate based on cost components.
| Hospital | Total NICU cost* | ECMO cost | Percent ECMO |
|---|---|---|---|
| A | $7,345,099 | $294,000** | 4.0 |
| B | $2,270,935 | $100,000** | 4.4 |
| C | $7,127,012 | $336,000** | 4.7 |
| D | $4,681,261 | $111,072*** | 2.4 |
| E | $2,528,801 | $141,097*** | 5.6 |
| Average % = 4.2 |
Table 6: Cost of Neonatal ECMO Cases Using Actual Total Charges*
*Only CHNMC includes physician fees.
**National Perinatal Information Center.
+Children's Hospital National Medical Center.
++1.15 is a conservative inflation factor.
" Based on number of cases in the group.
N/A=Not Available.
| n | All | n | Lived | n | Died | |
|---|---|---|---|---|---|---|
| NPIC** Hospitals (1988) |
12 | $111,295 | 10 | $95,905 | 2 | $188,248 |
| Ochsner (1988-1989) |
46 | $95,175 | 39 | $95,055 | 7 | $95,843 |
| University of Michigan (1985-1988) |
N/A | -- | 20 | $52,076 | N/A | -- |
| CHNMC+ (1985) |
25 | $91,804 | 20 | $98,320 | 5 | $65,740 |
| (1988 x 1.15)++ | $105,574 | $113,068 | $75,601 | |||
| Pooled" | 83 | $100,638 | 20 | $89,540 | 14 | $101,814 |
Table 7: Average Length of Life (ALOL) for Infants Who Died With "ECMO Diagnoses" 1984 Birth Cohort (by diagnosis)
| Number of cases | Percent of cases | ALOL | |
|---|---|---|---|
| Sepsis | 557 | 24.9 | 8.8 |
| Massive Aspiration Syndrome (MAS) | 496 | 22.1 | 6.1 |
| Congenital Diaphragmatic Hernia (CDH) | 417 | 18.6 | 6.7 |
| Severe Respiratory Distress Syndrome (RDS) | 359 | 16.0 | 16.2 |
| Pneumothorax PRI | 188 | 8.4 | 7.7 |
| Persistant Pulmonary Hypertension (PPHN)* | 166 | 7.4 | 44.2 |
| Bronchopulmonary Dysplasia (BPD) | 58 | 2.6 | 126.0 |
| All | 2,241 | 100.0 | 14.6 |
Table 8: Average Length of Life (ALOL), in Days, for Infants Who Die With "ECMO Diagnoses" 1984 Birth Cohort (by timing of death)
| Length of life | Number of cases | Percent of cases | ALOL |
|---|---|---|---|
| 1-23 hrs. | 609 | 27 | 1.0 |
| 1-6 days | 1,001 | 45 | 2.7 |
| 7-27 days | 426 | 19 | 13.1 |
| >28 days | 205 | 9 | 116.1 |
| All | 2,241 | 100 | 14.6 |
Beyond asking for budgeted amounts, we requested information on charges for services to estimate both the marginal cost per case and the operational costs of the unit. Table 3 shows the components of the analysis. In general, hospitals bill for NICU and PICU care in addition to a per diem charge for ECMO. These costs are billed together for each day on ECMO except on the first day, when a set-up fee for the ECMO procedure is charged and no ECMO per diem is charged. Disposables were reported as an average lump sum per case. As shown, the average cost for the ECMO-care component of treatment is $16,288, excluding physician fees and subsequent non-ECMO care days. The budgeted amounts and the calculated costs of ECMO are shown in Table 4. Using the different methods of calculating cost, the average of direct costs for ECMO on an operating basis ranges between $243,500 per year and $651,520, which breaks down to an average per case cost of between $6,087 and $16,288 for ECMO service. The figures based upon budgets and components do not include overhead, which makes up the largest part of the difference between these figures and those based on charges which are by their nature "marked-up" with an overhead rate.
In a vacuum, it is difficult to know whether these figures are over or under estimations. From the point of view of operations, it is important to know how they relate to total NICU costs. Table 5 shows the relationship between ECMO costs and total NICU costs for five hospitals. ECMO budgets represent between 2.4 percent and 5.6 percent of all NICU costs. The mean for the 5 hospitals was 4.2 percent. ECMO patients at these facilities represent roughly 3 percent of NICU admissions.
Average Total Acute Inpatient Cost Per Case for ECMO Patients and Conventional Care Patients
The actual acute inpatient cost per case incurred for patients who receive ECMO is simply the sum of the charges. This is also true for conventional care patients. Table 6 presents the charge experience for ECMO patients from 4 sources and pools the data. Cases who lived (89 cases) experienced a range of costs from $52,076 at the University of Michigan to a high of $113,068 at the Children's Hospital National Medical Center (CHNMC), with a pooled average of $89,540. The 14 cases who died had a much greater range, $75,601 to $188,248. The pooled mean for those who died was $101,814 or 13.7 percent above those who lived. When all cases are examined together (excluding those from University of Michigan), the pooled mean for 83 cases is $100,638 per ECMO case.
Conventional care cases also came from multiple sources. Part of the NPIC conventional-care data came from the 1984 birth-death cohort. There were 2,241 newborns in 1984 who died of ECMO diagnoses (see Table 7); they lived an average of 14.6 days before dying. Cases with PPHN lived the longest, an average of 44.2 days. While Bronchopulmonary Dysplasia (BPD) is not strictly an ECMO diagnosis, it is one which represents the outcome of cases potentially treatable by EMCO. BPD cases are included because they represent the largest proportion of ECMO eligible cases who live over 28 days and then die and therefore are the most expensive potentially preventable deaths. Sepsis, Massive Aspiration Syndrome (MAS) and Congenital Diaphragmatic Hernia (CDH) account for 65.6 percent of all cases and, on average, die within 1 week. Table 8 shows that 72 percent of all ECMO-eligible deaths die within 6 days, while only 9 percent die after 28 days.
On average, these newborns live 14.6 days before they die. This figure could be converted directly into cost per case based upon analysis of the 28-hospital data set. However, we decided to adjust the ALOL using distribution of cases by diagnosis reported by the ECMO Registry.17 The distribution and the results of the adjustment are shown in Tables 9 and 10, respectively. As can be seen, the ALOL drops from the 14.6 to 13.6 although MAS, CDH, and sepsis account for 69 percent of the cases. To ensure that our analyses would be conservative in subsequent calculations of the cost for the conventional care group, 14.6 is used as the ALOL for those who die but would have been eligible for ECMO.
We used the 28 tertiary hospital data base to estimate the cost of care for those newborns who died and to identify the cost of cases who lived with those diagnoses (see Table 11). Of the 87,915 cases in the data set, there were > 3,293 newborns over 2,000 grams with "ECMO diagnoses" of these, 3,220 lived and 73 died. The 73 who died had an average length of stay (ALOS) of 11.1 days and an average charge of $18,423. Those who lived had an ALOS of 9.1 days. This low ALOS indicates this group was less ill and required less care than newborns who typically receive ECMO. Without detailed medical record data, selecting the sickest of cases who were truly ECMO eligible from this group would be impossible. As a proxy, we selected the 11 most expensive cases on which we had complete data. The mean length of stay and charges for these cases was 36.7 days and $51,811, respectively.
Table 9: Distribution of 715 ECMO Cases 1980-1987* Submitted to the ECMO Registry
*Toomasean et al. Reference No. 14.
| Number of cases | Percent incidence | |
|---|---|---|
| MAS | 310 | 43 |
| CDH | 121 | 17 |
| PPHN/PFC | 100 | 14 |
| RDS | 96 | 13 |
| Sepsis | 64 | 9 |
| Cardiac | 12 | 2 |
| Other | 9 | <1 |
| Pneumothorax | 3 | <1 |
| Total | 715 | 100 |
Table 10: Adjusted Average Length of Life (ALOL) for Distribution of 100 Potential ECMO Cases Based on ECMO Registry Case Distribution 1980-1987
*Distribution of ECMO cases based on Table 9.
**Average length of life by diagnosis for infants who died with "ECMO diagnoses," from Table 7.
+PPHN was used as the diagnosis to ensure conservative calculations.
| Percent incidence* | ALOL** | |
|---|---|---|
| MAS | 43 | 6.1 |
| CDH | 17 | 6.7 |
| PPHN | 14 | 44.2 |
| RDS | 13 | 16.2 |
| Sepsis | 9 | 8.8 |
| Cardiac | 3 | N/A |
| Pneumothorax | 1 | 7.7 |
| Other+ | 1 | 44.2 |
| All | @ 100 | 13.6 |
The figures on charges were used in Table 12 to develop cost estimates for conventional-care patients. Note that rather than using the ALOS for the patients on the tertiary hospital data set, we used the ALOL from the birth cohort analysis and assumed that the per day costs were based upon those actually experienced by the 73 cases who died (18,422÷11.1= $1,660). The adjusted charge per case is $27,871 per newborn who died with conventional care (after inflation). The 11 most expensive cases had mean charges per case of $59,583.
Table 13 shows the figures from the analysis of the birth cohort and tertiary hospital data sets along with case data from CHNMC, University of Michigan, and Columbia-Presbyterian. Newborns who lived and who died are shown separately; weighted means were calculated to provide an overall grand mean for each. The average costs for a conventional-care survivor are $53,606; the average costs for those who die are $33,195. Comparing our findings with the cost of ECMO from Table 6, we find that conventional care costs are about 40 percent lower for those who live and 67 percent lower for those who die.
Discussion
These figures indicate that, at least for acute care on a per case basis, ECMO costs more. We realize that our conclusions run counter to those of Pearson and Roloff; however, our analysis uses a sample of the most expensive conventional care cases. Our estimates of the cost of ECMO are conservative (in part because physicians' costs are largely excluded); according to our more extensive data set, cost discrepancies may actually be even greater and in the opposite direction of those seen by other researchers. In estimating the system-wide costs, the disparity will be even greater because ECMO causes newborns to survive—a desirable outcome, but one resulting in greater hospital costs. The ECMO Registry indicates that in excess of 80 percent of those treated with ECMO live. Our data show that a "conventional care" death costs about one-third the cost of either an ECMO case who lives or who dies. If only 44 percent of cases who would have died in the United States of ECMO-related causes were treated with ECMO (1,006 cases treated in 1989 divided by 2,241 eligible ECMO cases who died), our health system costs would be at least $90,077,240 (1,006 x $89,540 assumes 100 percent live) compared to a cost of $40,170,212 if 80 percent of cases were to die with conventional care. This discrepancy would be less if we accept arguments (see James) that optimal conventional care allows survival rates substantially in excess of 20 percent.
Table 11: Tertiary Hospital Data Base (n=28 1985)
| Number of cases | Average length of stay | Average charge/case | Percent died | |
|---|---|---|---|---|
| All Cases | 87,915 | 5.8 | $3,260 | 1.4 |
| "ECMO Diagnoses">2000g | 3,293 | 9.1 | $7,417 | 2.3 |
| "ECMO Diagnoses">2000g—Survived | 3,220 | 9.1 | $7,168 | |
| "ECMO Diagnoses">2000g—Died | 73 | 11.1 | $18,423 | |
| "ECMO Diagnoses"—Most Costly Survivors | 11 | 36.7 | $51,811 |
Table 12: Cost of Comparison Cases, 1985 Adjusted to 1988
*National Perinatal Information Center
**Based upon charge per day of 73 cases with "ECMO diagnoses" who died in 1985 from 28-hospital random sample from Table 11.18,423¸ 11.1 = $1660/day.
***Based on the 11 most costly cases w/th the 7 "ECMO diagnoses" who lived from the 28-hosp/ta/ random sample.
+14.6/s used as a more conservative estimate of ALOL (i.e. longer than 11.1 days) and is based on the 1984 birth-death cohort presented in Tab/es 7 and 8.
++Inflation factor of 15% was used.
| NPIC* 1984 birth cohort | Average length of stay | Average daily charge | Actual Charges/case 1985 | Adjusted Charges/case ++ 1988 |
|---|---|---|---|---|
| Died (80%) | 14.6+ | $1,660** | $24,236 | $27,871 |
| Lived (20%) | 36.7 | $1,411 | $51,811*** | $59,583 |
| Weighted Mean | $29,750 | $34,212 |
Another approach to system costs relies on hospital-based marginal operating cost. If we use the average reported ECMO budget of $349,000 (from Table 4) and multiply by the number hospitals responding to the survey, then it would cost hospitals in 1989 $5,235,000 to care for 600 patients (15 hospitals x 40 which is the average number of patients seen) beyond the NICU costs already being incurred. This analysis assumes that without ECMO the 40 patients per facility would be absorbed within the NICUs existing resource constraints. If this figure is extrapolated to 57 hospitals, the figure is $19,893,000. We should note, however, that the costs hospitals pass on to the system in charges are not based on marginal operating cost but on patient charges.
System-wide acute costs are much less meaningful outside the context of a long-term perspective. A long-term perspective essentially places a societal value on survival based upon productive working years and, in some cases, the quality of life. Table 14 lists many of the factors essential to evaluating long-term cost per ECMO survivor compared to conventional approaches.
Table 13: Summary of All Conventional Care Cases (No ECMO)
*National Perinatal Information Center.
**Children's Hospital National Medical Center.
***See reference no. 6.
+ Personal communication, Dr. P. Bartlett.
++ Personal Communication, Dr. S. James. Total charge data based upon Columbia-Presbyterian NICU and ancillary charges, 1988.
+++ University of Michigan had 2 cases who died but no information on cost or length of stay was available.
" 54 cases were used here rather than 73 to keep the ratio of lived to died equal to the expected number of based on the national demand and to ensure a more conservative final figure.
Survivors Only
| Number of cases | Average length of stay | Average charge/case | |
|---|---|---|---|
| NPIC* (1988) | 11 | 36.7 | $59,583 |
| CHNMC** (1984-1985)*** | 54 | 75.8 | $173,282 |
| University of Michigan+ | 275 | 34.0 | $67,058 |
| Columbia Presbyterian++ (1983-1985) | 27 | 14.2 | $30,951 |
| Mean for survivors | 47 | 26.8 | $53,606 |
Died Only +++
| Number of cases | Average length of stay | Average charge/case | |
|---|---|---|---|
| NPIC | 54" | 14.6 | $27,871 |
| CHNMC | 10 | 22.0 | $61,620 |
| Columbia Presbyterian | 2 | 1.0 | $1,650 |
| Mean for died | 66 | 15.3 | $32,190 |
Even if we were to accept the figures presented here as scientific estimates of the cost of treatment (and as noted earlier, we have excluded the physician component for ECMO almost entirely), we would still need to know the cost of subsequent medical care (inpatient and outpatient), the average life expectancy of survivors over childhood (short term) and into adulthood (long term), whether or not morbidity is such that these survivors lives would be valued as normal pro ductive working individuals who pay taxes and who require the normal amount of education without special non-medical interventions to promote their growth and development. Finally, based upon morbidity, we would need to make assumptions about expected lifetime earnings. To our knowledge, little of the long-term information is available. Even the most basic assumption, mortality within the first year of life on conventional care, is in dispute.18
Table 14: Factors Important to Lifetime Saving or Cost Per Survivor
- More accurate cost for ECMO treatment
- Cost of subsequent medical care
- Average life expectancy of survivors (short- and long-term mortality)
- Disability/morbidity rates
- Cost of education for survivors
- Expected lifetime earnings for those able to work
It is not unusual in our society to pay for care that is not strictly cost effective. Table 15 shows the number of infants in the extremely low birth-weight categories and the average charges per case to care for these newborns in the acute inpatient phase of care. There were 7,888 500—750 gram infants born in 1984, of whom roughly 25 percent lived, who had charges on average of $119,476. Those who died had average charges of $12,479. While the survival rate is better for infants 750—999 grams, the 1985 acute-care charge averaged $81,839. Most recently Faranoff and Hack19 noted that progress in the treatment of infants <750 grams or 26 weeks gestation has not improved. Similarly, Walker et al. found that treating infants under 900 grams at birth was not justifiable from a cost-benefit standpoint.20 Boyle21 demonstrated similar findings for infants under 1,000 grams. These findings, however, have not affected our continued treatment of very-low-birthweight infants.
Neonatal care is unique in medicine in its reliance on technology and its placement of a high regard on life with little emphasis on cost. While it is important to understand costs, our society historically and currently does not take cost into account when deciding on what is an established treatment. Table 16 shows information presented in this paper and compares frequency, cost, and mortality of ECMO to liver transplantation, as documented in a recent government report.22 Liver transplants have increased dramatically, with one-third of all cases to date occurring in 1987 (most recent year reported). Costs are higher than those for ECMO and survival is lower. The survival beyond 5 years is unreported. Liver transplantation—considered an established treatment—occurs in older people, requires more complex surgery, requires an organ donation, and an entire system of organ support for the procedure to occur with any regularity. The potential system costs exceed those for ECMO, both because it is more expensive and because there are potentially many more eligible candidates. Our upside estimate of demand for ECMO was 1 case for every 1,309 births, or 2,801 per year, a ratio which produces somewhat higher estimates than those of Southgate23 which were 1 case for every 1,707, despite our using a very similar methodology. There are about 4,600 persons per year who may be acceptable liver transplant candidates. Nonetheless, it is extremely difficult to single out any one technology in a policy vacuum and identify it as prohibitively expensive.
So far we have discussed only questions of cost. Medical technologies are generally evaluated first in terms of benefits. This important question regarding ECMO as opposed to conventional care has been debated (see James in this publication). Another critical policy question is the matching of supply of a new technology to true demand.
Table 15: Resource Use Among 500—999 Gram Birthweight Infants 1984—1985
*Analysis of the 1984 birth cohort National Center for Health Statistics, Sept. 1989.
**From 28 tertiary hospital database (1985).
NCHS Cohort*
| Total Born | Died | Percent Died | |
|---|---|---|---|
| 500-750g | 7,888 | 5,899 | 74.7 |
| 750-999g | 8,927 | 3,578 | 40.1 |
28 Hospital Cohort**
| Survived Cases | Survived ALOS | Survived Avg. Chgs. | Died Cases | Died ALOS | Died Avg. Chgs. | |
|---|---|---|---|---|---|---|
| 500-750g | 74 | 1,12.9 | $119,476 | 273 | 8.7 | $12,479 |
| 750-999g | 242 | 82.6 | $81,839 | 14.8 | $22,777 |
Table 16: Comparison of ECMO and Another Expensive Technology—Liver Transplants
*Figures from reference no. 22.
**Varies by diagnosis.
| ECMO (1989) | Liver Transplant* (1987) | |
|---|---|---|
| Number of U.S. Centers in Operation | 57 | 50 |
| Cases to Date | 3,593 | 3,500 |
| Cases in Most Recent Year | 1,006 | 1,187 |
| Expected Mortality Without Treatment | 80% | 100% |
| Reported Cost Per Case (range) | $52,076-$188,248 | $150,000-$259,000 |
| 1-Year Survival | 83%** | 69%** |
| 5-Year Survival | N/A | 60%** |
We estimate outside demand at 2,801 cases per year, of which 1,001 were handled in 1989 within the existing centers. In addition, it appears that not all centers are operating at optimal capacity. The 15 centers responding to our survey reported the ability to care for an average of 2.9 cases simultaneously. With an average length of stay of 6.0 days, these hospitals could each care for an average of 136 more cases per hospital (above the mean of 40) with existing equipment and space. Given these assumptions, the 15 respondant hospitals have the capacity to care for an additional 1,950 cases without expansion (at 100 percent occupancy). Even if we assume that problems of queuing and staffing drop optimal occupancy to 50 percent, the units could care for an additional 975 cases. There are, however, an additional 42 hospitals providing ECMO who have not been surveyed, who cared for the remaining 400 cases in 1989, but who have an unknown capacity. This obviously does not include the planned expansion by another 31 hospitals.
Expansion will add additional units to the system without a clear need and at a probable increment to hospitals' NICU budgets of between 2.4 and 5.6 percent above current NICU costs. Expanding supply beyond demand may create pressures to expand the entry criteria for use of the technology, thereby further increasing cost over conventional care without established benefit. Without making additional calculations, it appears that the expansion is unwarranted in terms of demand and potentially may create pressures for care beyond standards historically accepted for treatment using strict mortality criteria, thereby undermining the accepted notion of optimal care.
From a policy perspective, not only are guidelines concerning who should provide care essential,24 but so are guidelines indicating the optimal number of cases per year to ensure both the quality of care and its economic provision.
Our conservative estimate, based upon the largest data bases analyzed for these issues, indicates that, whatever its benefits to newborns, ECMO is substantially more expensive than conventional care. Policy decisions regarding new technologies are not made on cost alone; here ECMO's benefit for survival is the central question. Even if ECMOs benefits are in the range of current estimates, creation of additional ECMO units may be unwarranted based upon limited demand.
References
- Snedoor SM: ELSO, Neonatal Registry Report, Ann Arbor, Ml Ibid.
- 1988-1989 Amer can Hospital Association Survey of Obstetrical Hasp
faa, sponsoren by the Maternar and Goila Healfo Section of AHA in collaboration with NPIG and NGHS. - 1988-1989 Neonatal ECMO Patients From Ochaner Hospital, provided by Br. Robert Arenaman.
- Ralaff BW of al.: A Prospective Randomized Study a/Goat-Effectiveness. Dopublished (with special permission of Br. Robert Bartlett).
- Pearson 0, Short BL: An economic analysis of extracorpatea membrane oxygenation. Intensive Gate Medicine 2:116-120,1987.
- IGB-GM-9 /nfernafional 0 assificafion of Diseases 9fn Rev sian GImbal Modification, vo . 1, GPHA, Ann ArDor, Ml, 1978.
- ORouke PP of al.: Extracorporea membrane oxygenation and conventional men cab therapy in neonates with persistent pulmonary hypertension of the newborn: A prospective randomizen study. Pediatrics 84:957-963, 1989. Ba. Barf[eff of al.: Extracorporeal circulation in neonatal
respiratory/a l..re: A prospect ye rannomizen stony. Pediatrics 76:479-487, 1985. - Pearson, op cit.
- Rob/f, unpublished op cit.
- James SL: Neonatal Problems Treated With ECMO: Pathology Prevent on Alternative Therapies, presented at Workshop on toe Diffusion of ECMO Technology, May 31— June 1,1990.
- Loked Birth/Infant Death Ba/a Set: 1984 Birth Gohort. 0.5. Department of Heal/n ann Human Services, Genters for Disease Gonfrol, ann National Genfer for Health Statistics, September, 1989.
- ELSO, op c.f
- Toomasian JM of ab.: National experience ext racarporeab membrane oxygenation for newborn respiratory failure: Data from 715 cases. ASAIO 34:140-147, 1988.
- Schwartz RM, Oagnon BE, Mon JH of ab.: BROS and Their Impact an Perinatab Beg ona zafion. Sponsored by the Maternal and Go d Health Research Program, Project OMGJ-440525-03, August, 1989.
- Schwartz RM: What price premafurty? Family Planning Perspectives 21:4,1989.
- Tanmasian, op cit.
- Wang JT, of ab.: Management of infants with severe respiratory failure and persistence of foe fetal circulation without nypervenfibat on. Pediatrics 76:4, 488-494, 1985.
- Hack M, Faranaff AA: Outcomes n/extremely law bitt hweight infants between 1982 and 1988. N EngI J Med 321:1642-1647, 1989.
- Walker BJ ef ab.: Goat benefit analysis of neonatal intensive care far infants weigning less toan 1000 grams at birth. Pediatrics 74:20-25, 1984.
- Boyle MB of al.: Econamo evaluation a/neonatal intensive care a/very law birfoweight infants. N EngI J Med 308:1330-1337, 1983.
- Hat/a 55: Health Teconalogy Assessment Reports. Assessment of Liver Transplantation. 0.5. BHHS, PBS, AHGPP, 1990.
- Sau/hgafe WM, Howell GO, Kanta WP, Jr. at al.: Too need far and impact on neonatal mortality of oxtracarporeal membrane axygenafan in in/ants a/greater than 2500 gram birth weight. Pediatrics 86:71-4, 1990.
- committee an Fetus ana Newborn: Recommendatans an extracarpareal membrane oxygenation. Pediatrics 85:61 8- 619, 1990.
 BACK TO TOP
BACK TO TOP