Findings may inform methods to make new antibiotics and introduce medications into cells
Credit: National Institutes of Health
Researchers at the National Institutes of Health believe they have discovered how influenza viruses open a hole in the cell membrane to inject genetic material into the cell. The findings may inform the development of new technologies to combat infectious microbes and to insert medications, genes, and proteins into cells to treat diseases.
The study was conducted by first author Amy Rice, Ph.D., senior author Joshua Zimmerberg, Ph.D., and colleagues at NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Heart, Lung and Blood Institute and at the Russian Academy of Sciences. It appears in Nature Communications.
Background
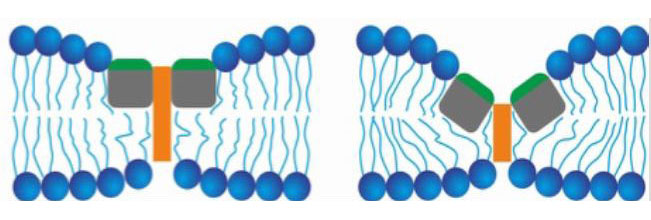
Cells are enveloped in a membrane that protects from disease-causing organisms, while permitting nutrients to enter and waste to leave. To infect a cell, spike proteins—projections on the virus’s outer surface—bind to the cell’s surface. The cell’s outer membrane envelops the virus in a pocket-like structure called a vacuole, which encapsulates the virus and takes it inside the cell.
Through a series of experiments, computer simulations, and mathematical models, the researchers investigated the sequence of events occurring after the vacuole takes the virus into the cell.
Results
The researchers found that within the acidic environment of the vacuole, fusion peptides on the spike protein bind to the vacuole’s inner membrane. The membrane is composed of two lipid (fatty) layers. Fusion peptides bind to the inner lipid layer, and adjacent fusion peptides bind to each other. This attraction of the fusion peptides pulls membrane proteins together and pushes away the fats, thinning the membrane at the binding site. A rocking motion of the peptides penetrates the outer layer of the membrane, thinning the spot to the point of breaking, as the action pinches the inner and outer lipid layers together.
Significance
The researchers believe that these molecular interactions can explain why so many viruses make holes in their target membranes, why the fusion machinery of influenza variants typically does not mutate, and how spike proteins allow viral genetic material to enter the cell.
Reference
Rice, A, et al. Planar aggregation of the influenza viral fusion peptide alters membrane structure and hydration, promoting poration. Nature Communications. 2022.

 BACK TO TOP
BACK TO TOP