Delivery of the gene for neurotrophic factor-α1 (NF-α1) directly into the hippocampus of the brain prevents neurodegeneration and memory loss in a mouse model of Alzheimer’s disease, according to a study led by NIH researchers. These preclinical findings on a potential therapeutic are among the first to show prevention of Alzheimer’s disease in an animal model. The results are published in the latest issue of Molecular Psychiatry.
Background
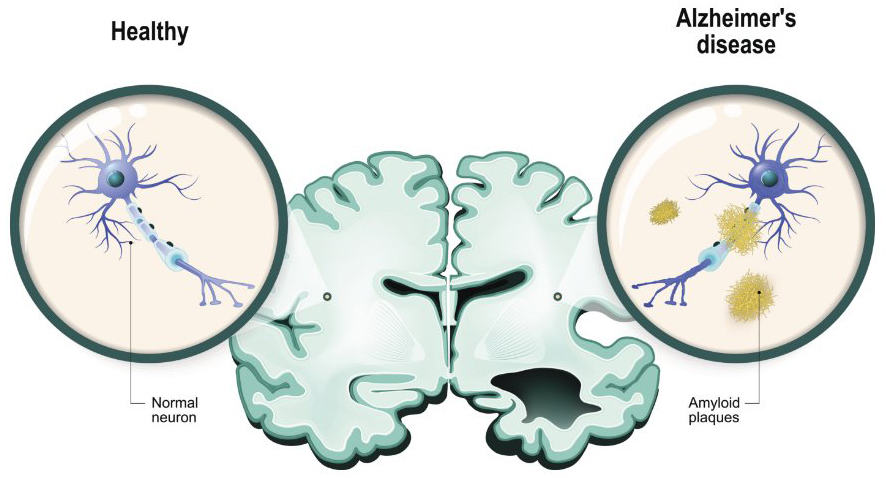
Alzheimer’s disease is the most common cause of dementia among older adults. The disease often occurs in people 65 years or older and progressively causes decline in memory, thinking skills, and more. It is not a typical part of the aging process. In a small subset of patients, Alzheimer’s disease is genetically inherited, and symptoms occur in early adulthood. Furthermore, many people with Down syndrome go on to develop Alzheimer’s disease as they age. Therefore, the development of Alzheimer’s disease likely results from several different factors, complicating the development of potential therapies.
To date, many experimental therapies target only one component of Alzheimer’s disease, for example, clearing amyloid plaques in the brain, with limited success. Previous work from the Loh Lab at NICHD has identified a neuroprotective role for NF-α1 (initially called carboxypeptidase E [CPE]), particularly against stress. In the current study, the team followed up on these findings by testing whether the addition of NF-α1 to the hippocampus, an area of the brain involved in memory, via gene therapy can prevent or lessen symptoms of Alzheimer’s disease.
Results
The study team used a mouse model of Alzheimer’s disease called 3xTg-AD, where symptoms and changes in the brain are apparent by age six months. They used gene therapy to introduce extra copies of the NF-α1 gene into both sides of the hippocampus in two-month-old mice before symptoms develop. The researchers then tested the mice at age seven to eight months to evaluate their cognition, memory, and other neurological factors.
The team found that NF-α1 gene therapy prevents development of Alzheimer’s disease. Mice treated with NF-α1 displayed no learning and memory deficits when undergoing a standard water maze test. Mice treated with NF-α1 also seemed to retain their memory skills during an object recognition test. Analysis of brain samples also indicated that NF-α1 treated mice did not experience neurodegeneration or other common symptoms of the disease.
Importantly, the researchers found that NF-α1 decreased the production of amyloid precursor protein (APP) in the brain, which contributes to the plaque formation that is a hallmark of Alzheimer’s disease. None of the other factors tested (NGF, BDNF, or FGF2), while having some effect on improving cognition, had this ability to prevent plaque formation. Therefore, NF-α1 works by preventing the formation of plaques, rather than clearing them away.
Significance
The study is among the first to show prevention of Alzheimer’s disease progression in an animal model, likely by targeting multiple causes that contribute to the disease. The findings may also be applicable to Down syndrome, where excessive plaque formation by APP causes early-onset Alzheimer’s disease.
Next Steps
While the preclinical animal testing is promising, more research is needed to test NF-α1—its safety and efficacy, as well as partnering with industry to explore non-invasive means of delivery.
Reference
Xiao L et al., Hippocampal delivery of neurotrophic factor-α1/carboxypeptidase E gene prevents neurodegeneration, amyloidosis, memory loss in Alzheimer’s disease male mice. Molecular Psychiatry DOI: 10.1038/s41380-023-02135-7 (2023)

 BACK TO TOP
BACK TO TOP