Genetic disorder alters blood vessel formation in the eyes, brain, neck, and spine
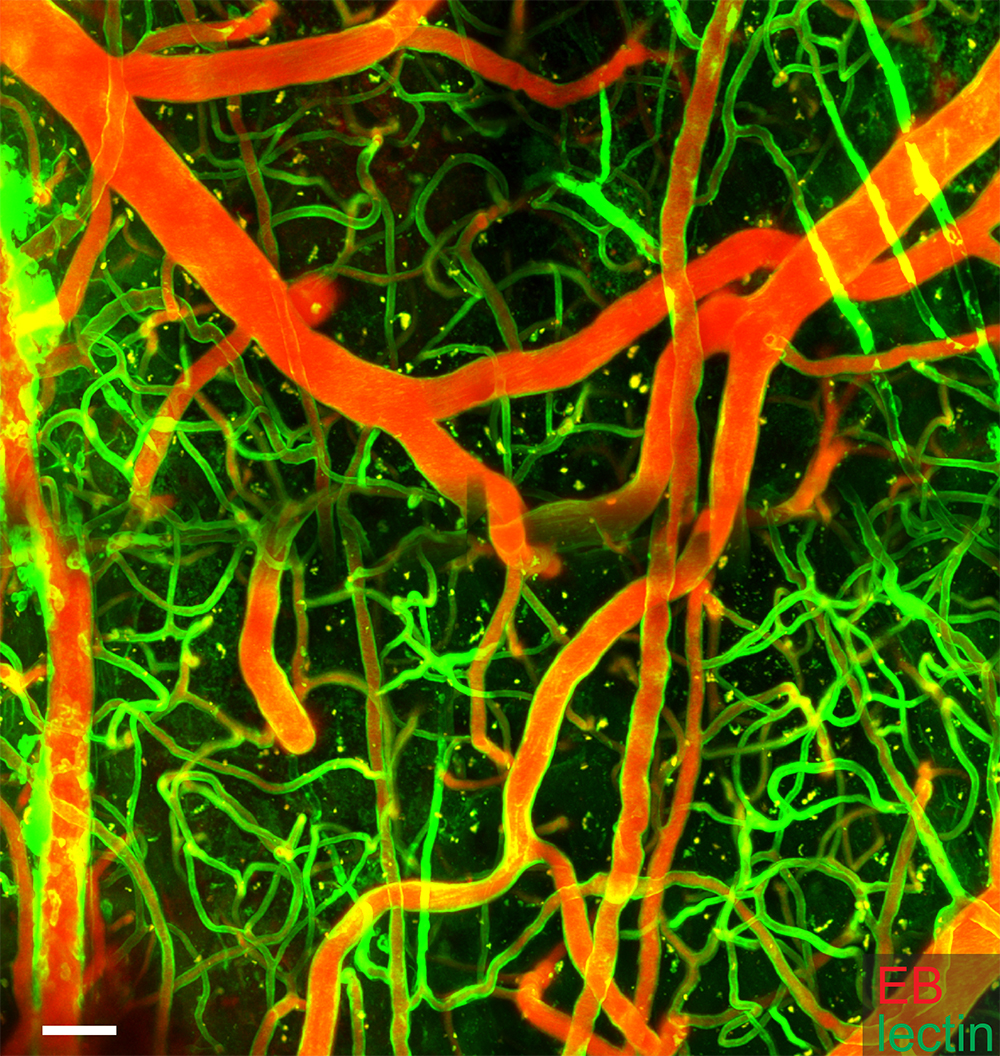
Credit: Drs. Jared Rosenblum, Karel Pacak, et al.
NIH researchers have identified additional symptoms of EPAS1 gain-of-function syndrome, a rare disease resulting in hormone-secreting tumors and an increase in red blood cells. In a group of 9 patients and in a mouse model of the disease they developed, researchers observed malformations of blood vessels and related structures in the eyes, brain, neck, and spine.
The study was led by Karel Pacak, M.D., D.Sc., Ph.D., a senior investigator in the Section on Medical Neuroendocrinology at NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and Jared S. Rosenblum, M.D., a research fellow at NIH’s National Cancer Institute. The study appears in JCI Insight.
Background
EPAS1 gain-of-function syndrome was first reported in 2012 by Dr. Pacak and his collaborators. It is characterized by neuroendocrine tumors (arising from cells of the nervous system that secrete hormones), such as paraganglioma and somatostatinoma. The disease results from a mutation in the EPAS1 gene, which disrupts the function of its corresponding protein, hypoxia-inducible factor-2α (HIF-2α).
HIF-2α senses oxygen and coordinates cellular responses when oxygen is low. The protein is controlled by oxygen; when oxygen levels return to normal, HIF-2α is degraded because it is no longer needed. However, in EPAS1 gain-of-function syndrome, HIF-2α fails to degrade and does not respond to oxygen changes.
This rare disease provides an opportunity to better understand the role of HIF-2α, which is involved in normal development, blood vessel formation, immune responses and inflammation, and the development of tumors, which usually have lower levels of oxygen compared to surrounding tissue.
Results
The study team used imaging methods, such as MRI and CT, to evaluate vessels and structures in the patients and the mouse model. Unlike neuroendocrine tumors, which are thought to develop later in life, the researchers found developmental malformations that would have been present at birth. They reported a number of blood vessel-related problems in the eyes, brain, neck, and spine of patients as well as in the mouse model.
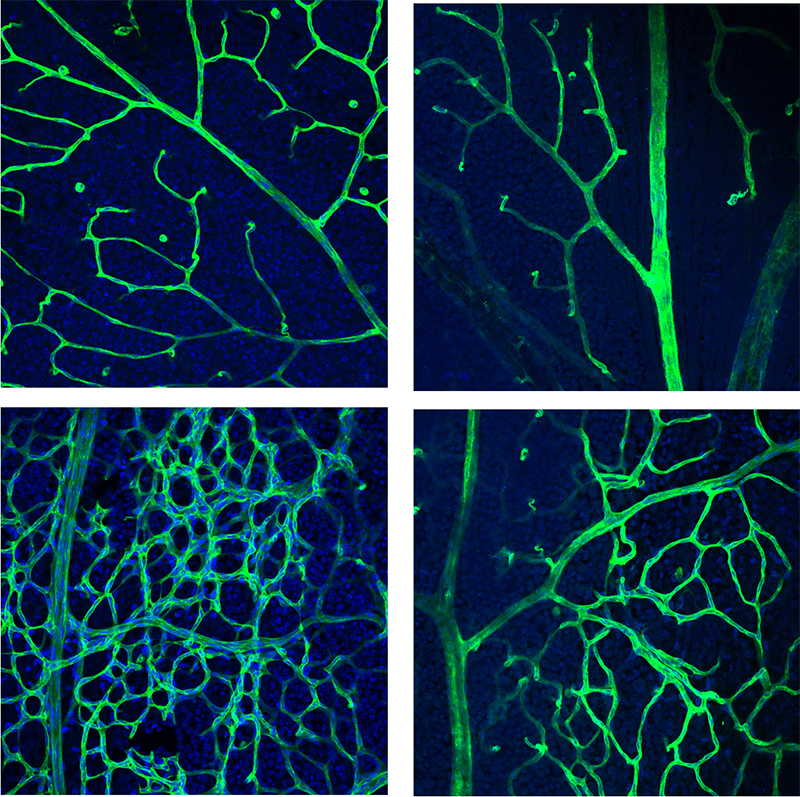
Credit: Drs. Jared Rosenblum, Karel Pacak, et al.
The mutation resulted in networks of excessive blood vessels in various tissues. The researchers also found that in patients, these vessels had a higher frequency of the mutation than the surrounding tissue. The researchers believe that this mutation in EPAS1 prevents the normal pruning of blood vessels 
“This finding of congenital malformations brings us closer to understanding the development of sporadic vascular malformations,” said Dr. Rosenblum.
Significance
“Providers should be aware that patients with EPAS1 gain-of-function syndrome may present with symptoms, such as impaired vision, that are caused by stable, congenital problems present since birth,” added Dr. Pacak. “Refining our understanding of this rare disease can help us better treat and manage the condition over time.”
Next Steps
The study team will conduct additional studies using the mouse model to learn how these malformations may form in the body. Understanding these mechanisms could lead to new or better treatments.
Reference
Rosenblum JS et al., Developmental vascular malformations in EPAS1 gain-of-function syndrome. JCI Insight DOI: 10.1172/jci.insight.144368 (2021)

 BACK TO TOP
BACK TO TOP