Richard Levine key to effort linking blood compounds to serious pregnancy disorder
An NIH scientist whose landmark collaborations led to a major advance in understanding a potentially fatal disorder of pregnancy has passed away.
 Richard J. Levine, M.D., M.P.H., died of lymphoma at the age of 71. He is survived by his wife, Verena, and two daughters, Adele and Nicole.
Richard J. Levine, M.D., M.P.H., died of lymphoma at the age of 71. He is survived by his wife, Verena, and two daughters, Adele and Nicole.
Dr. Levine was best known for his pivotal role in a sequence of studies. This effort established that two substances found in the blood are largely responsible for the complex of symptoms in preeclampsia. In essence, the substances act as decoys, depriving blood vessels of essential compounds, in the process setting the stage for the sometimes dangerously high blood pressure and other symptoms that occur in the disorder.
“To those who worked with him, Dr. Levine was a kind and patient mentor who had a gift for planning studies and for framing scientific questions,” said Alan E. Guttmacher, Director of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). “To all who knew him, he was first and foremost a good friend.”
Dr. Levine was a senior investigator in the NICHD’s Division of Epidemiology, Statistics, and Prevention Research.
“Dr. Levine’s NICHD career was an example in patience, persistence, and seizing opportunity,” Dr. Guttmacher added. “Soon after he joined the institute, he undertook a large study to test a potential treatment for preeclampsia. When the study showed the treatment didn’t work, he didn’t simply move on to other areas. Rather, he held the biological samples from the study in storage for possible future use. Years later, he learned about the work of a promising young researcher who had a new theory of preeclampsia. Dr. Levine then placed a shrewd bet. He forged a collaboration with the young scientist, contributing the stored samples to the effort. The two went on to confirm the theory and establish the biochemical basis for the high blood pressure central to the disorder.”
Preeclampsia is a life-threatening pregnancy complication that affects 3 to 5 percent of pregnancies. Along with sometimes dangerously high blood pressure, preeclampsia also results in the appearance of protein in the urine. In the United States, the condition is rarely fatal, but can lead to lasting health problems resulting from damage to the liver and kidneys or from stroke or hemorrhage. The high blood pressure central to preeclampsia can quickly and unexpectedly lead to eclampsia—seizures that progress to coma and even death. In the developing world, where advanced medical care often is not available, preeclampsia is a leading cause of maternal death, killing as many as 75,000 women a year.
The only known cure for preeclampsia is to deliver the baby. If preeclampsia appears toward the end of a pregnancy, labor can be induced early, when the risk for newborn complications of premature birth is low. However, preeclampsia early in the pregnancy presents a serious challenge. Deliver the baby early, and increase the risk for such complications of prematurity as cerebral palsy, intellectual disability, blindness, and infant death. Allow the pregnancy to proceed, and increase the mother’s chances of seizure, coma, or death.
Dr. Levine joined the NICHD’s Division of Epidemiology, Statistics, and Prevention Research in 1991. Previously, he was an epidemiologist for the Chemical Industry Institute of Toxicology, where he studied male fertility. He also held positions with the U.S. Centers for Disease Control and Prevention and with the Alabama Department of Public Health.
After joining the NICHD, Dr. Levine undertook a major project to learn whether women could reduce their chances of developing preeclampsia by taking calcium supplements. Earlier studies and several meta-analyses suggested that calcium might protect against the disorder. Dr. Levine and colleagues at the NICHD and five hospitals undertook the Calcium for Preeclampsia Prevention (CPEP) trial. From 1992 to 1995, the study enrolled 4,589 healthy first time mothers who were from 13 to 21 weeks pregnant. About half (2,295 women) were randomly assigned to take four calcium tablets per day, with each tablet containing 500 mg. of calcium. The remaining 2,294 women took a placebo containing no calcium. The researchers collected detailed medical histories from all the participants. They also collected blood and urine samples from the women at three intervals in their pregnancies.
When the study ended, the researchers found no significant differences between the two groups. In the July 7, 1997 issue of the New England Journal of Medicine, they reported that calcium did not prevent preeclampsia in the first-time mothers who took part in the study.
Although the study failed to show any benefit of calcium in preeclampsia, Dr. Levine had the foresight to save the stored samples. He made the samples available for a couple of research collaborations, but the results weren’t especially promising.
In the summer of 2002 Dr. Levine received a manuscript from a colleague. In the article, S. Ananth Karumanchi, M.D., a young researcher at Beth Israel Deaconess hospital in Boston outlined a theory on the biochemical basis of preeclampsia. Dr. Karumanchi and his coauthors described a series of experiments they had conducted on a substance found in placentas of women with preeclampsia. Dr. Levine was so impressed that he read the paper twice.
“And I thought it was the best thing that I had ever read,” he said in an article that appeared in The New Yorker in 2006.
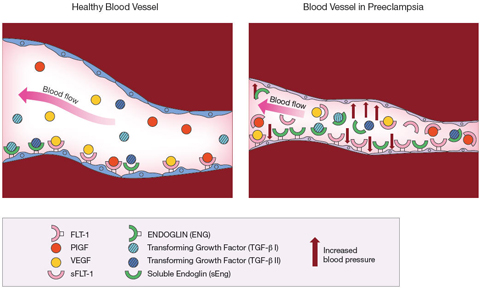
The substance, soluble fms-like tyrosine kinase 1 (sFlt-1), was present in much higher levels in the blood of women with preeclampsia than in women who did not have the disorder. This molecule was a variant of Flt-1, a free-floating version of the molecule normally found on the inner lining of blood vessels. Flt-1 is a receptor— a docking point for other molecules. The molecules— placental growth factor (PlGF) and vascular endothelial growth (VEGF)— attach, or bind, to Flt-1. The binding process is necessary for the blood vessels to remain healthy.
The study later appeared in the March, 2003 Journal of Clinical Investigation. The researchers linked higher levels of the freely circulating sFlt-1 to preeclampsia. They theorized that sFlt-1 acted as a decoy, capturing PlGF and VEGF so that they couldn’t dock with the Flt-1 receptors on the blood vessel walls. Deprived of PlGF and VEGF, the blood vessels deteriorated. According to the theory, this blood vessel deterioration was the basis for the high blood pressure and other symptoms of preeclampsia.
In support of the theory, Dr. Karumanchi and his colleagues analyzed blood samples of about 30 women, two thirds of whom had preeclampsia. The researchers found that sFlt-1 was present in higher levels in the blood of women with preeclampsia than in the blood of women with normal pregnancies. At the same time, the women with preeclampsia had lower blood levels of PlGF and VEGF.
Evidence from the laboratory also supported the theory. Rodent blood vessels incubated in serum from the women with preeclampsia soon deteriorated, becoming frayed and generally less healthy than vessels incubated in serum from pregnant women who did not have preeclampsia. Adding PlGF and VEGF to the serum restored the frayed vessels to normal. Similarly, pregnant rats exposed to high levels of sFlt-1 soon developed the high blood pressure and protein in the urine seen in preeclampsia.
Using the stored samples from Dr. Levine’s earlier calcium study, the two began a collaborative effort to test the theory. In their first effort, they showed that, compared to women who did not have preeclampsia, a group of 120 women with preeclampsia had elevated levels of sFlt-1 and correspondingly low levels of PlGF and VEGF. In a 2004 New England Journal of Medicine article, the researchers also showed that the levels of sFlt-1 began climbing about 5 weeks before the women developed preeclampsia.
“Richard had a very clear sense of purpose,” Dr. Karumanchi said. “He was an extremely careful epidemiologist who knew how to design a study to answer important questions. Yet he never forgot—and wouldn’t let anyone else forget—that we were in this to help people.”
In the next analysis, Drs. Karumanchi, Levine, and their coworkers uncovered more support for the theory. They found that levels of PlGF in the urine of women who later developed preeclampsia began dropping by the 25th week of pregnancy.
Efforts are under way by other researchers to develop a test to predict the onset of preeclampsia, based on levels PlGF in the urine.
Although the studies of Drs. Karumanchi and Levine confirmed that sFlt-1 played a major role in preeclampsia, these studies did not establish that the sFlt-1 accounted for all cases of the disease. Some women with high sFlt1 levels did not develop preeclampsia, whereas others with low levels did develop the condition.
Dr. Karumanchi reviewed the findings from his first analysis of placeplacentas. He found that a second substance, soluble endoglin (sEng), also was present in placentas from women with preeclampsia. As with Flt-1 and sFlt-1, free circulating and fixed forms of the molecule were found in blood vessels. The Eng receptor was a docking point for a molecule known as transforming growth factor beta (TGF beta), also needed to keep blood vessels healthy. Again, the free circulating sEng acted as a decoy, diverting TGF beta away from the blood vessels. Deprived of TGF beta, blood vessels become less elastic.
In September 2006, the researchers published a study showing that testing for sEng as well as sFlt-1 accounted for all cases of preeclampsia among the women in the CPEP study. Among the women who developed preterm preeclampsia, levels of soluble endoglin began increasing in the 17th through the 20th week of pregnancy. In women who developed preeclampsia near the end of their pregnancies, soluble endoglin levels rose at the 25th to the 28th week of pregnancy.
“Both soluble endoglin and the altered sFlt1/PlGF ratio appear to contribute to the development of preeclampsia,” Dr. Levine said in an NIH news release describing the study. “Severe disease usually occurs in women with high levels of both measures and not in women with high levels of only one or the other.”
After the study results were published, Dr. Levine continued to forge new collaborations involving the CPEP samples. One such effort found that women with a history of preeclampsia are at risk for thyroid disease later in life. The thyroid gland, located in the front of the throat, makes hormones that help regulate heart rate, blood pressure, body temperature, and the conversion of food into energy. Reduced thyroid functioning, or hypothyroidism, results in overall weakness and fatigue and also increases the risk for cardiovascular disease. High levels of TSH indicate that the thyroid may not be producing enough hormones.
The study, which appeared in the British Medical Journal in 2009, combined two separate analyses to reach its conclusion. In the first, Drs. Levine, Karumanchi, and their colleagues analyzed CPEP samples and found that, on average, women with preeclampsia had elevated levels of thyroid stimulating hormone (TSH). Because the CPEP study did not provide information on thyroid functioning after the women’s pregnancies, the researchers analyzed another set of data. That study matched pregnancy and birth records from women who had given birth in 1967 to results of thyroid function tests the same women underwent in the mid 1990s. Women who had preeclampsia in their first pregnancy were 1.7 times more likely to have high TSH levels than were the women who did not have preeclampsia. Women who had preeclampsia in both their first and second pregnancies were nearly 6 times more likely to have high TSH levels.
The two continued their collaboration until about a week before Dr. Levine’s death. In their most recent effort, they submitted two papers for publication on the role of blood vessel factors in birth complications of preterm infants born to women with preeclampsia.
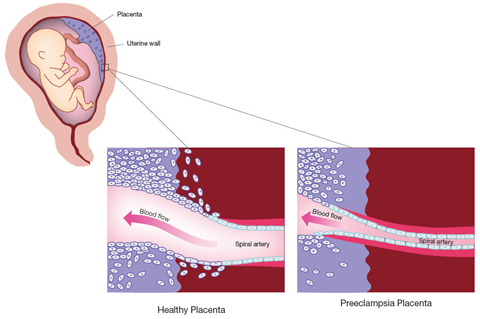
The studies involving the CPEP samples account for the symptoms experienced by mothers with preeclampsia. But the abnormalities seen in the placenta during preeclampsia have not been explained. In a normal pregnancy, finger-like projections of placental cells invade the uterine blood vessels, mimicking the cells lining blood vessels. In the process, they widen the uterine blood vessels, increasing blood flow to the placenta. A 1997 study funded by the NICHD found that, in preeclampsia, this process of invasion and widening appears to stop early. The connections between the maternal and fetal circulation remain narrow and largely undeveloped, and the blood supply to the placenta is restricted.
One theory is that preeclampsia results when the placenta adapts to the restricted blood flow by pumping sFlt-1 and sEng into the maternal circulation. The increased pressure is thought to increase blood flow to the placenta.
Rodney E. Kellems, M.D., a preeclampsia researcher at the University of Texas Medical School, recalls seeing Dr. Levine two years ago, at a conference on hypertension in pregnancy, reading the abstracts in the scientific poster session.
“He was working his way through the posters,” Dr. Kellems said. “He looked frail, and obviously tired. Someone offered him a chair, and he took it. But he didn’t stop. He just sat down, and continued reading the posters.”
At the poster session, Dr. Levine was intrigued by work done by Dr. Kellems’ University of Texas colleague, Yang Xia, M.D. Ph.D. With funding from the NICHD’s Pregnancy and Perinatology Branch, Dr. Xia had been investigating the role of an antibody found in the blood of women with preeclampsia. Antibodies are Y-shaped molecules produced by the immune system. Like guided missiles, antibodies seek out, and stick to, molecules on the surface of disease causing organisms, marking them for destruction by the immune system.
Earlier, researchers discovered that women with preeclampsia had a special type of antibody. This antibody sought out, and stuck to, a receptor on the surface of the body’s own cells, the angiotensin II receptor. Although this antibody stuck to the receptor, it didn’t mark the receptor for destruction. Instead, the antibody stimulated the receptor. When the antibodies attached themselves to the angiotensin II receptors on heart cells from mice, the cells began beating faster.
Studies led by Dr. Xia found that when the antibody bound to receptors of cells in laboratory cultures, the antibody caused the cells to produce sFlt-1 as well as a number of other chemicals compounds that affected blood vessels. Further experiments showed that the antibody bound to cells in the kidneys, to cells lining the insides of blood vessels, and to cells of the placenta. When the antibody was injected into pregnant mice, the mice soon developed high blood pressure, protein in the urine, and other symptoms of preeclampsia. In fact, the antibody could cross the placenta and reach the mouse fetus. They later tested 37 women with preeclampsia and found that all the women had the antibody in their blood. Moreover, levels of the antibody correlated with the severity of the women’s illness. Those with the most severe cases had the highest antibody levels. Similarly, they also found the antibody in the umbilical cord blood of women with preeclampsia who had given birth, which indicated that the antibody had crossed the placenta and entered the fetal circulation.
The researchers theorize that the stunted, abnormal placenta seen in preeclampsia doesn’t cause the disorder but may be a casualty of it. Dr. Xia’s theory holds that, for reasons not well understood, the mother produces antibodies to the angiotensin II receptor. The antibodies cross into the placenta and stimulate the placental cells to produce sFlt-1, which, along with other compounds from the placenta, enter the mother’s circulation, hinder the placenta’s development, and then trigger the high blood pressure and other symptoms of the disease. In laboratory studies, the researchers found they could block the antibody, and prevent it from reaching the angiotensin II receptor. It follows that it may be possible to treat preeclampsia by preventing the antibody from binding to the receptor.
Drs. Levine and Xia soon began corresponding. Although his health was failing, he found time to begin one more collaboration, to place one more bet.
He provided Dr. Xia and her colleagues with 1000 samples from the CPEP study, which had been collected at three intervals throughout the women’s pregnancies. Dr. Xia and her colleagues are about to begin analyzing the samples for the antibody.
###
About the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
The NICHD sponsors research on development, before and after birth; maternal, child, and family health; reproductive biology and population issues; and medical rehabilitation. For more information, visit the Institute’s Web site at http://www.nichd.nih.gov/.

 BACK TO TOP
BACK TO TOP