Discovery provides new target for anti-malaria treatments
Credit: National Institutes of Health
Click to view image.
WHAT:
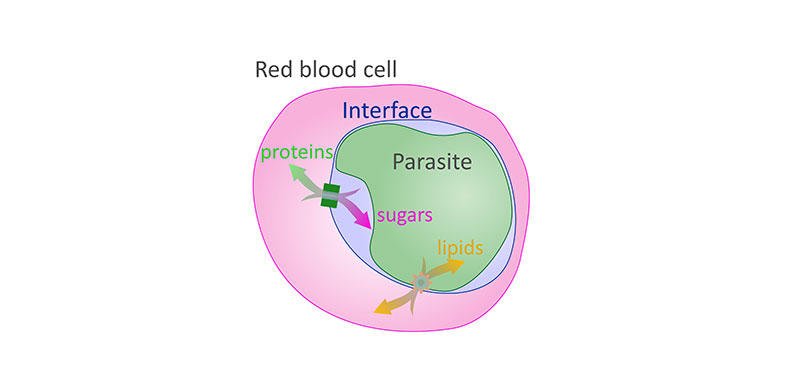
Researchers at the National Institutes of Health and other institutions have discovered another set of pore-like holes, or channels, traversing the membrane-bound sac that encloses the deadliest malaria parasite as it infects red blood cells. The channels enable the transport of lipids—fat-like molecules—between the blood cell and parasite, Plasmodium falciparum. The parasite draws lipids from the cell to sustain its growth and may also secrete other types of lipids to hijack cell functions to meet its needs.
The finding follows an earlier discovery of another set of channels through the membrane enabling the two-way flow of proteins and non-fatty nutrients between the parasite and red blood cells. Together, the discoveries raise the possibility of treatments that block the flow of nutrients to starve the parasite.
The research team was led by Joshua Zimmerberg, M.D., Ph.D., a senior investigator in the Section on Integrative Biophysics at NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The study appears in Nature Communications.
In 2018, there were 228 million cases of malaria worldwide, leading to more than 400,000 deaths, 67% of which were among children under 5, according to the World Health Organization 
WHO:
Joshua Zimmerberg, M.D., Ph.D., of the NICHD Section on Integrative Biophysics is available for comment.
ARTICLE:
Garten et al. Contacting domains segregate a lipid transporter from a solute transporter in the malarial host-parasite interface. Nature Communications. 2020.
###
About the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): NICHD leads research and training to understand human development, improve reproductive health, enhance the lives of children and adolescents, and optimize abilities for all. For more information, visit https://www.nichd.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit https://www.nih.gov.

 BACK TO TOP
BACK TO TOP