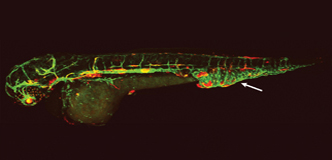
Spotlight: Genetic Research Offers Hope for Targeted Therapies for Lymphatic Malformations

Explore the Bethesda campus, including the NICHD Zebrafish Research Section (Building 6), and learn more about how NIH works to turn discovery into health.

NICHD provides highlights of some of our COVID-19 research activities to understand the effects of the virus among the populations central to our mission.

Through STRIVE, NICHD aims to improve equity, diversity, and inclusion in its workforce and scientific endeavors.

April 8, 2024
Infertility affects millions of lives. NICHD supports research to better understand its causes and contributing factors and to improve treatments for both male and female inferility.
Active Funding Announcements
Find a Program Officer
Small Business Programs
 BACK TO TOP
BACK TO TOP